Comparison of DRV/r + 3TC vs DRV/r + 3TC/TDF in ANDES Study
"ANDES Study compares the efficacy and safety of DRV/r + 3TC dual therapy versus DRV/r + 3TC/TDF standard therapy in ARV-naive patients, showing non-inferior virologic results but differences in adverse events. Explore details here."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
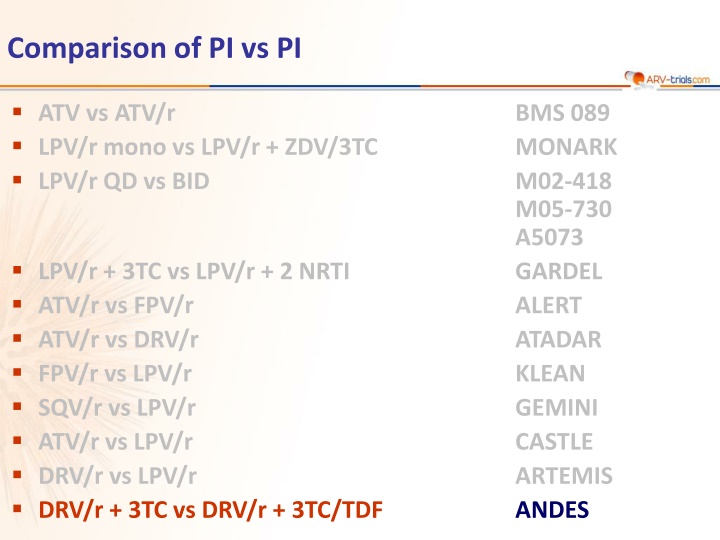
Comparison of PI vs PI ATV vs ATV/r LPV/r mono vs LPV/r + ZDV/3TC LPV/r QD vs BID BMS 089 MONARK M02-418 M05-730 A5073 GARDEL ALERT ATADAR KLEAN GEMINI CASTLE ARTEMIS ANDES LPV/r + 3TC vs LPV/r + 2 NRTI ATV/r vs FPV/r ATV/r vs DRV/r FPV/r vs LPV/r SQV/r vs LPV/r ATV/r vs LPV/r DRV/r vs LPV/r DRV/r + 3TC vs DRV/r + 3TC/TDF
ANDES Study: DRV/r + 3TC vs DRV/r + 3TC/TDF Design Randomisation* 1 : 1 Open-label W48 N = 75 > 18 years ARV-na ve DRV/r 800/100 mg FDC + 3TC 300 mg QD HIV RNA > 1 000 c/mL Any CD4 cell count HBsAg negative No R to study drugs N = 74 DRV/r 800/100 mg FDC + 3TC/TDF QD *Randomisation was stratified by HIV RNA (< or > 100 000 c/mL) at screening Objective Non inferiority of DRV/r + 3TC at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis Figueroa MI, CROI 2018, Abs. 489 ANDES
ANDES Study: DRV/r + 3TC vs DRV/r + 3TC/TDF Baseline characteristics and patient disposition DRV/r + 3TC N = 75 DRV/r + 3TC/TDF N = 74 Median age, years 30 30 Female, % 7 12 HIV RNA (log10 c/mL), median 4.6 4.5 HIV RNA > 100 000 c/mL, % 27 22 CD4 cell count (/mm3), median 419 367 Discontinuation by W24, N Adverse event Lost to follow-up Withdrew consent 4 2 1 1 1 0 1 0 Figueroa MI, CROI 2018, Abs. 489 ANDES
ANDES Study: DRV/r + 3TC vs DRV/r + 3TC/TDF Efficacy outcome at W48 DRV/r + 3TC N = 75 DRV/r + 3TC/TDF N = 74 Difference : - 1.0 % (95% CI : - 7.5 to 5.6) HIV RNA < 50 c/mL, % 93 94 HIV RNA < 50 c/mL in patients with baseline HIV RNA > 100 000 c/mL, % Median CD4 increase (/mm3) 91 92 246 200 p = 0.20 Safety at W48 DRV/r + 3TC N = 75 DRV/r + 3TC/TDF N = 74 Grade 2-4 adverse events possibly/probably related, N 11 17 Gastro-intestinal adverse events, % Rash, % Total cholesterol elevation LDL-cholesterol elevation Triglycerides elevation No treatment-related serious adverse event 7% 8% 19% 14% 25% 14% 7% 4% (p = 0.01) 6% (ns) 14% (ns) Figueroa MI, CROI 2018, Abs. 489 ANDES
ANDES Study: DRV/r + 3TC vs DRV/r + 3TC/TDF Summary DRV/r + 3TC dual therapy was virologically non inferior to a standard therapy of DRV/r + 3TC/TDF in na ve patients Similar virologic response of the 2 regimens in patients with HIV RNA > 100 000 c/mL at enrolment Only 1 case of virologic failure (DRV/r + 3TC/TDF) with no emergence of resistance Incidence of gastro-intestinal adverse events higher in triple therapy group Lipid elevations higher in the DRV/r + 3TC group, significantly higher than in DRV/r + 3TC/TDF group for total cholesterol Figueroa MI, CROI 2018, Abs. 489 ANDES