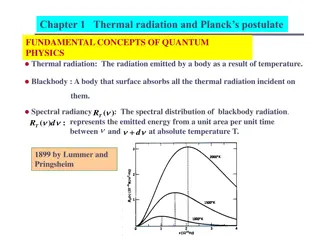
Comparison of Rayleigh-Jeans Law with Wien Approximation and Planck's Law
"Explore the comparison of Rayleigh-Jeans law, Wien approximation, and Planck's law at 8 mK temperature. Understand how classical physics and quantum mechanics intersect in describing black-body radiation."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Comparison of RayleighJeans law with Wien approximation and Planck's law, for a body of 8 mK temperature.
Comparison of RayleighJeans law with Wien approximation and Planck's law, for a body of 8 mK temperature. In physics, the Rayleigh Jeans law attempts to describe the spectral radiance of electromagnetic radiation at all wavelengths from a black body at a given temperature through classical arguments. For wavelength , it is: where c is the speed of light, kB is the Boltzmann constant and T is the temperature in kelvins. For frequency , the expression is instead The Rayleigh Jeans law agrees with experimental results at large wavelengths (low frequencies) but strongly disagrees at short wavelengths (high frequencies). This inconsistency between observations and the predictions of classical physics is commonly known as the ultraviolet catastrophe,[1][2] and its resolution was a foundational aspect of the development of quantum mechanics in the early 20th century. https://upload.wikimedia.org/wikipedia/commons/thumb/7/72/RWP-comparison.svg/300px-RWP-comparison.svg.png
Comparison to Planck's law In 1900 Max Planck empirically obtained an expression for black-body radiation expressed in terms of wavelength = c/ (Planck's law): where h is the Planck constant and kB the Boltzmann constant. The Planck law does not suffer from an ultraviolet catastrophe, and agrees well with the experimental data, but its full significance (which ultimately led to quantum theory) was only appreciated several years later. Since, then in the limit of very high temperatures or long wavelengths, the term in the exponential becomes small, and the exponential is well approximated with the Taylor polynomial's first-order term, So, This results in Planck's blackbody formula reducing to
This results in Planck's blackbody formula reducing to which is identical to the classically derived Rayleigh Jeans expression. The same argument can be applied to the blackbody radiation expressed in terms of frequency = c/ . In the limit of small frequencies, that is This last expression is the Rayleigh Jeans law in the limit of small frequencies.
which is identical to the classically derived RayleighJeans expression. The same argument can be applied to the blackbody radiation expressed in terms of frequency = c/ . In the limit of small frequencies, that is h \nu \ll k_\mathrm{B} T , B_\nu(T) = \frac{2h\nu^3}{c^2}\frac{1}{e^\frac{h\nu}{k_\mathrm{B} T} - 1} \approx \frac{2h\nu^3}{c^2} \cdot \frac{k_\mathrm{B} T}{h\nu} = \frac{2 \nu^2 k_\mathrm{B} T}{c^2}. This last expression is the Rayleigh Jeans law in the limit of small frequencies.
Consistency of frequency and wavelength dependent expressions When comparing the frequency and wavelength dependent expressions of the Rayleigh Jeans law it is important to remember that , and Therefore, even after substituting the value , because has units of energy emitted per unit time per unit area of emitting surface, per unit solid angle, per unit wavelength, whereas has units of energy emitted per unit time per unit area of emitting surface, per unit solid angle, per unit frequency. To be consistent, we must use the equality where both sides now have units of power (energy emitted per unit time) per unit area of emitting surface, per unit solid angle. Starting with the Rayleigh Jeans law in terms of wavelength we get
where . This leads us to find: