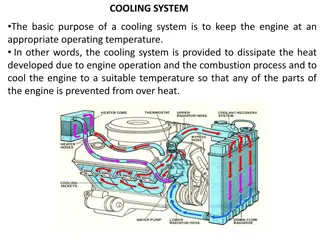
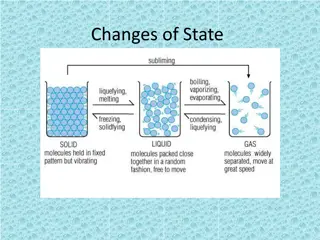
Condensation: Gas Cooling to Liquid
Condensation occurs when a gas cools and transforms into a liquid. This fundamental process plays a vital role in various applications, including weather phenomena and industrial systems. Understanding condensation's principles enables us to comprehend the transition of states and harness its practical implications effectively. Exploring its mechanisms sheds light on how temperature fluctuations impact matter, leading to the formation of liquid droplets. Through this process, we unveil the intricate dynamics of gas particles converting into a condensed state, shaping our surroundings and scientific endeavors.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Condensation To know that condensation is the process of gas cooling to form a liquid
So what is Condensation? Condensation is a process, a change It s the cooling process that changes gas into a liquid It is the opposite of evaporation.
Particles again folks! Air particles Water particles Dry day Wet day
Dry day On a dry day there is only a little water vapour in the air
Wet day On a damp day there is much more water vapour in the air
Bathroom mirror After a shower your bathroom is filled with steam (water vapour) from the hot water. When it hits the cold glass of the mirror it turns back to water and forms tiny droplets H e l l o ! H e l l o !
Look at the can What has happened? Why has it happened? What do we call the process?
Look at the glass of ice What has happened? Why has it happened? What do we call the process?
Look at mirror on top of the hot drink What has happened? Why has it happened? What do we call the process?
Breath on your mirrors What has happened? Why has it happened? What do we call the process?
09-10-06 Condensation To know that condensation is the process of gas cooling to form a liquid Task 1 choose the best word to complete these sentences 1. Condensation happens when water vapour cools/heats 2. Condensation is the opposite of dissolving/evaporation 3. On a wet day there are more air particles/water particles in the air
Next Illustrate what happens when steam from a kettle hits a cold window Useful words water vapour -steam gas cools evaporation process -liquid -heats -condensation -changes
Extension Explain in your own words what happens on a cold day when you breath on a cold window List other examples of condensation
What have you learnt? What is condensation the process of ? Can you give 3 examples of condensation happening?