Coordination Modes and Metal-Arene Complex Synthesis
Arenes coordinate in a 6-fold fashion with metals, forming stable complexes. The synthesis of metal-benzene complexes involves specific reactions and limitations. The properties of metal-bis(arene) complexes vary, showing distinct colors and behaviors. Backbonding plays a crucial role in arene-metal interactions. Electron counting and ligand coordination affect complex stability. Analyze the scenario for strong coordination of benzene ligands in different metal complexes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
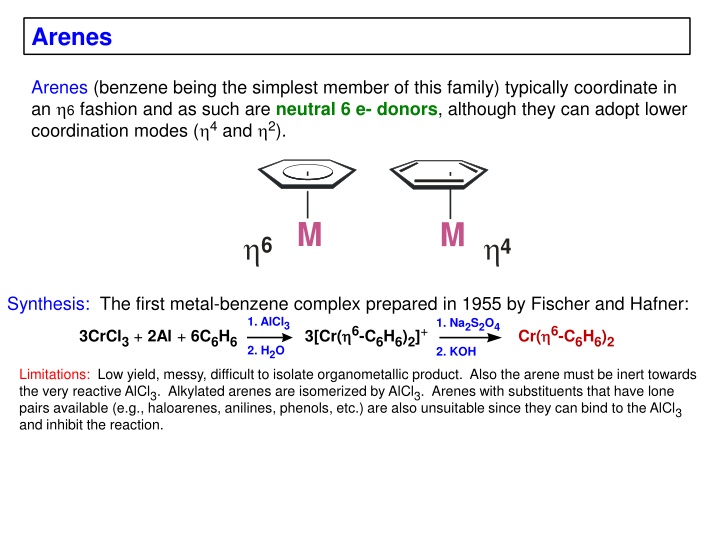
Arenes Arenes (benzene being the simplest member of this family) typically coordinate in an 6 fashion and as such are neutral 6 e- donors, although they can adopt lower coordination modes ( 4 and 2). M M 6 4 Synthesis: The first metal-benzene complex prepared in 1955 by Fischer and Hafner: 1. AlCl3 1. Na2S2O4 3CrCl3+ 2Al + 6C6H6 3[Cr( 6-C6H6)2]+ Cr( 6-C6H6)2 2. H2O 2. KOH Limitations: Low yield, messy, difficult to isolate organometallic product. Also the arene must be inert towards the very reactive AlCl3. Alkylated arenes are isomerized by AlCl3. Arenes with substituents that have lone pairs available (e.g., haloarenes, anilines, phenols, etc.) are also unsuitable since they can bind to the AlCl3 and inhibit the reaction.
Some Properties of Metal-bis(arene) Complexes Complex Color mp/ C Miscellaneous air-sensitive, autocatalytic decomposition in aromatic solvents very air-sensitive, paramagnetic, reducible to [V(C6H6)2] Ti(C6H6)2 red - V(C6H6)2 red 227 V(C6H5F)2 red - air-sensitive very air-sensitive, paramagnetic, decomposes at ca. 90'C air-sensitive, the cation [Cr(C6H6)2]+ is air-stable. E = 0.69 V in DME against SCE Nb(C6H6)2 purple - Cr(C6H6)2 brown 284 Mo(C6H6)2 green 115 very air-sensitive W(C6H6)2 yellow-green 160 less air-sensitive than Mo(C6H6) [Mn(C6Me6)2]+ pale pink - diamagnetic reducible to [Fe(C6Me6)2]+, violet, and to Fe(C6Me6)2, black, paramagnetic, extremely air-sensitive air-stable, diamagnetic; reducible to Ru(C6Me6)2, orange, diamagnetic, very air-sensitive Paramagnetic; reducible to Co(C6Me6)2, very air-sensitive [Fe(C6Me6)2]2+ orange - [Ru(C6Me6)2]2+ colorless - [Co(C6Me6)2]+ yellow -
-Backbonding -backdonation plays a relatively important role in arene bonding and chemistry. Arenes tend to favor metals in low oxidation states and often generate surprisingly stable complexes. Cr(C6H6)2, for example, is kinetically inert to most substitution reactions, no doubt due to its 18 e- configuration, but also due to the mix of - bonding and backbonding. Remember that CO and NO+ are far, far stronger -backbonding ligands. Problem: The crystal structure of [Cr(C6H6)2]+ clearly shows that the hydrogen atoms on the benzene distinctly lean in towards the metal center. Explain why.
A dramatic example of the power of the 18e- electronic configuration is seen for [Ru(C6Me6)2]2+ . This can be reduced to neutral Ru(C6Me6)2, but electron- counting with two 6-C6Me6 ligands gives you a 20e- complex. Me Me 6 Me Me Me Me Ru Me Me 4 Me Me Me
Problem: In which of the following complexes should the 6-benzene ligand coordinate the strongest? Why?? A) B) Mo Cr OC (MeO)3P CO P(OMe)3 OC (MeO)3P C) W Me3P PMe3 Me3P