Coregulatory Mechanism of DdrR on UmuDAb in SOS Mutagenesis
Investigating the coregulatory role of the DdrR protein on UmuDAb control of SOS mutagenesis in Acinetobacter baumannii. The study explores the interactions between these proteins and their impact on DNA damage repair mechanisms. Mutant strains were constructed to assess the contributions of DdrR to DNA binding, repression actions, and self-cleavage of UmuDAb. Results suggest that DdrR may affect UmuDAb repression activities, highlighting potential mechanisms of action in the context of mutagenesis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
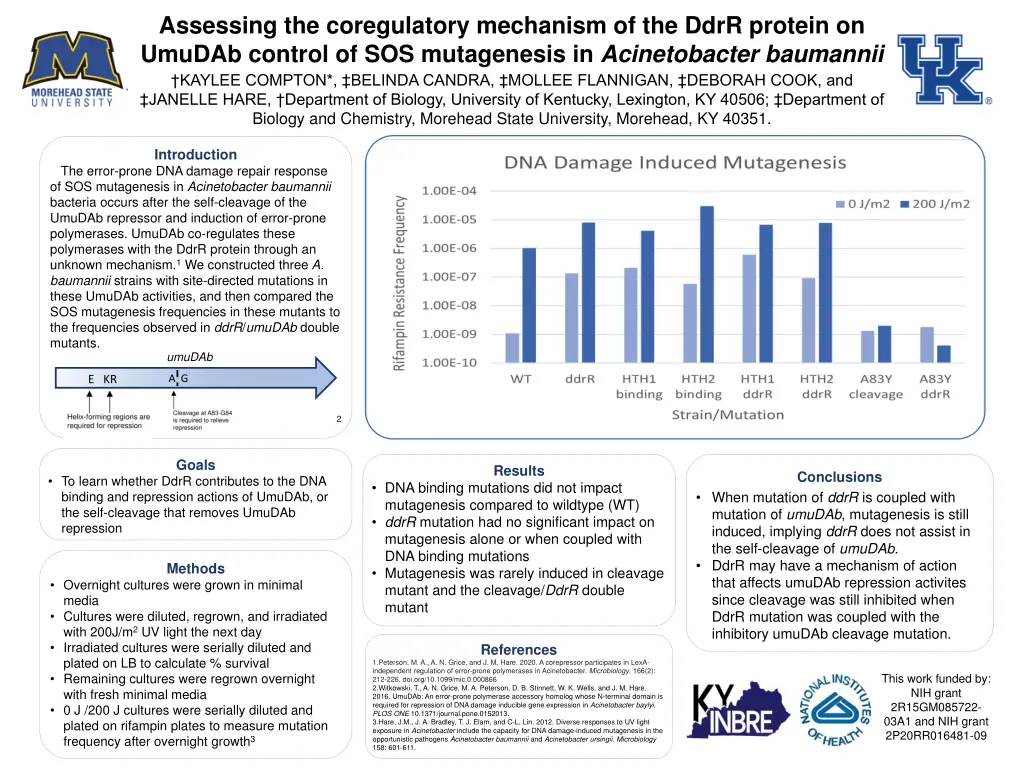
Assessing the coregulatory mechanism of the DdrR protein on UmuDAb control of SOS mutagenesis in Acinetobacter baumannii KAYLEE COMPTON*, BELINDA CANDRA, MOLLEE FLANNIGAN, DEBORAH COOK, and JANELLE HARE, Department of Biology, University of Kentucky, Lexington, KY 40506; Department of Biology and Chemistry, Morehead State University, Morehead, KY 40351. Introduction The error-prone DNA damage repair response of SOS mutagenesis in Acinetobacter baumannii bacteria occurs after the self-cleavage of the UmuDAb repressor and induction of error-prone polymerases. UmuDAb co-regulates these polymerases with the DdrR protein through an unknown mechanism.1 We constructed three A. baumannii strains with site-directed mutations in these UmuDAb activities, and then compared the SOS mutagenesis frequencies in these mutants to the frequencies observed in ddrR/umuDAb double mutants. umuDAb A G E KR 2 Goals Results Conclusions To learn whether DdrR contributes to the DNA binding and repression actions of UmuDAb, or the self-cleavage that removes UmuDAb repression DNA binding mutations did not impact mutagenesis compared to wildtype (WT) ddrR mutation had no significant impact on mutagenesis alone or when coupled with DNA binding mutations Mutagenesis was rarely induced in cleavage mutant and the cleavage/DdrR double mutant When mutation of ddrR is coupled with mutation of umuDAb, mutagenesis is still induced, implying ddrR does not assist in the self-cleavage of umuDAb. DdrR may have a mechanism of action that affects umuDAb repression activites since cleavage was still inhibited when DdrR mutation was coupled with the inhibitory umuDAb cleavage mutation. Methods Overnight cultures were grown in minimal media Cultures were diluted, regrown, and irradiated with 200J/m2 UV light the next day Irradiated cultures were serially diluted and plated on LB to calculate % survival Remaining cultures were regrown overnight with fresh minimal media 0 J /200 J cultures were serially diluted and plated on rifampin plates to measure mutation frequency after overnight growth3 References 1.Peterson, M. A., A. N. Grice, and J. M. Hare. 2020. A corepressor participates in LexA- independent regulation of error-prone polymerases in Acinetobacter. Microbiology. 166(2): 212-226. doi.org/10.1099/mic.0.000866 2.Witkowski, T., A. N. Grice, M. A. Peterson, D. B. Stinnett, W. K. Wells, and J. M. Hare. 2016. UmuDAb: An error-prone polymerase accessory homolog whose N-terminal domain is required for repression of DNA damage inducible gene expression in Acinetobacter baylyi. PLOS ONE 10.1371/journal.pone.0152013. 3.Hare, J.M., J. A. Bradley, T. J. Elam, and C-L. Lin. 2012. Diverse responses to UV light exposure in Acinetobacter include the capacity for DNA damage-induced mutagenesis in the opportunistic pathogens Acinetobacter baumannii and Acinetobacter ursingii. Microbiology 158: 601-611. This work funded by: NIH grant 2R15GM085722- 03A1 and NIH grant 2P20RR016481-09
Introduction The error-prone DNA damage repair response of SOS mutagenesis in Acinetobacter baumannii bacteria occurs after the self-cleavage of the UmuDAb repressor and induction of error-prone polymerases. UmuDAb co- regulates these polymerases with the DdrR protein through an unknown mechanism.1 We constructed three A. baumannii strains with site-directed mutations in these UmuDAb activities, and then compared the SOS mutagenesis frequencies in these mutants to the frequencies observed in ddrR/umuDAb double mutants. umuDAb E KR A G 2
Goals To learn whether DdrR contributes to the DNA binding and repression actions of UmuDAb, or the self-cleavage that removes UmuDAb repression
Methods Overnight cultures were grown in minimal media Cultures were diluted, regrown, and irradiated with 200J/m2 UV light the next day Irradiated cultures were serially diluted and plated on LB to calculate % survival Remaining cultures were regrown overnight with fresh minimal media 0 J /200 J cultures were serially diluted and plated on rifampin plates to measure mutation frequency after overnight growth3
Results DNA binding mutations did not impact mutagenesis compared to wildtype (WT) ddrR mutation had no significant impact on mutagenesis alone or when coupled with DNA binding mutations Mutagenesis was rarely induced in cleavage mutant and the cleavage/DdrR double mutant
Conclusions When mutation of ddrR is coupled with mutation of umuDAb, mutagenesis is still induced, implying ddrR does not assist in the self-cleavage of umuDAb. DdrR may have a mechanism of action that affects umuDAb repression activites since cleavage was still inhibited when DdrR mutation was coupled with the inhibitory umuDAb cleavage mutation.
References 1.Peterson, M. A., A. N. Grice, and J. M. Hare. 2020. A corepressor participates in LexA-independent regulation of error-prone polymerases in Acinetobacter. Microbiology. 166(2): 212-226. doi.org/10.1099/mic.0.000866 2.Witkowski, T., A. N. Grice, M. A. Peterson, D. B. Stinnett, W. K. Wells, and J. M. Hare. 2016. UmuDAb: An error-prone polymerase accessory homolog whose N- terminal domain is required for repression of DNA damage inducible gene expression in Acinetobacter baylyi. PLOS ONE 10.1371/journal.pone.0152013. 3.Hare, J.M., J. A. Bradley, T. J. Elam, and C-L. Lin. 2012. Diverse responses to UV light exposure in Acinetobacter include the capacity for DNA damage-induced mutagenesis in the opportunistic pathogens Acinetobacter baumannii and Acinetobacter ursingii. Microbiology 158: 601-611. This work funded by: NIH grant 2R15GM085722-03A1 and NIH grant 2P20RR016481-09





















