Covalent Bonds and Their Properties
Explore the nature of covalent bonding, why atoms form bonds, and the properties of covalent compounds. Learn about the shared electrons, stability, energy involvement, and intermolecular forces impacting covalent molecules.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
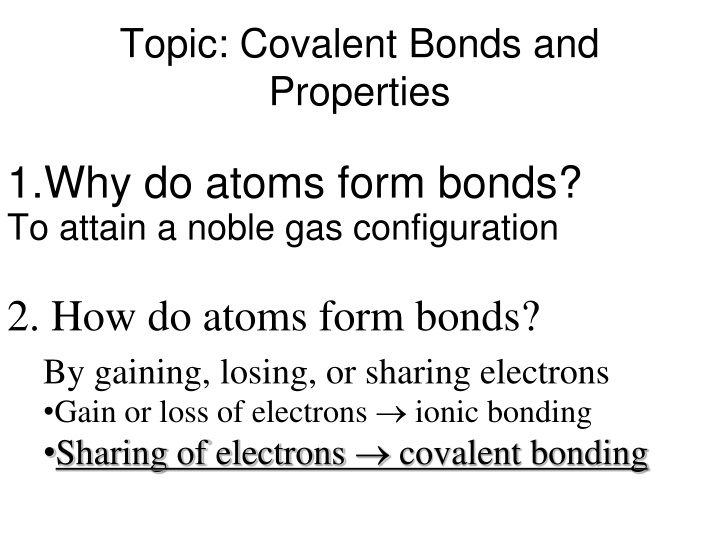
Topic: Covalent Bonds and Properties 1.Why do atoms form bonds? To attain a noble gas configuration 2. How do atoms form bonds? By gaining, losing, or sharing electrons Gain or loss of electrons ionic bonding Sharing of electrons covalent bonding
REVIEW Forming a bond = more stable = releases energy A + B AB + energy Breaking a bond = elements by themselves are less stable = requires energy CD + energy C + D
Review: Ionic Compounds made of ions Form crystal lattice + - + All are solid at room temp - - + - Ion is surrounded by 6 opposing ions So, strong electrostatic attraction - + - + Thus, high MP/BP, low VP
Covalent Bonding Results from Nonmetals ONLY There is an electrostatic attraction between nucleus (protons) one atom & electrons of neighbor s atom Electrons are shared
Compounds with covalent bonds are molecular! covalent compounds are often called molecules
Structure of Covalent Compounds NOT necessarily empirical - a lot are molecular .we can keep adding atoms! glucose C6H12O6 lipids (fats) empirical
Covalent Molecules are held together by IMF IMF holds molecules together IMF can be dispersion (nonpolar) Dipole-dipole (polar) Remember IMF determines phase H-bond (polar)
weakest IMF = dispersion forces - occur between nonpolar molecules Monatomic molecules: He, Ne, Ar, Kr, etc. Diatomics of same element: O2, H2, N2, etc Pure Hydrocarbons: CxHy Small Symmetric molecules: CO2, CCl4 Dispersion forces as size molecule
Properties of Covalent Molecules Depend on strength of IMF between particles or separate units (molecules)
Properties of Covalent (Molecular) Substances are determined by IMF between the molecules Poor conductors of heat & electricity (no charged particles!) Low mp & low bp easy to pull molecules apart from each other Majority of solids are soft Low Hf and Hv compared to ionic & metallic substances High VP compared to ionic & metallic substances
mp, bp, Hf and Hv and vapor pressure depend on how hard it is to pull particles apart Weak IMF easy to pull particles apart Strong IMF more difficult to pull apart
Which substance has the strongest intermolecular forces? The weakest? Water Ether
Another Type of Covalent Bonding: Coordinate Covalent Bonding = Polyatomic Ions from Table E bond formed when: 2 atoms share pair electrons but both electrons donated by same atom many polyatomics have coordinate covalent bonds must be able to recognize them
Ammonium, NH4+1 H.. .. + H : N : H H+1 no electrons at all! 2 electrons just hanging around
Ammonium, NH4+1 +1 H.. .. H : N : H H Both electrons in this bond were donated by the N atom
Hydronium, H3O+1 .. + H : O : H .. H+1 2 pairs of nonbonding electrons zero electrons
Hydronium, H3O+1 +1 H.. Both electrons are provided by the oxygen atom H : O : H ..
Another Type of Covalent Bonding: Network Covalent Bonding Atoms (often Si or C) covalently bonded to one another THEY MAKE A LATTICE Example: SiO2 (sand), Diamonds (C), Graphite (C) They are very hard, Low Vp, High MP Not soluble in water It networks that s where it gets it s strength (so diff. properties)
Carbon = forms networks, like above and in organic chemistry it can also form long chains and rings
