COVID-19 Vaccine Updates & Trials Summary
This update provides information on the latest COVID-19 vaccine candidates - Moderna, AstraZeneca & University of Oxford, BioNTech & Pfizer. Details include trial phases, side effects, immune response, and potential timelines. Stay informed about the progress of these promising vaccines in combating the virus.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
COVID 19 Vaccine update
Agenda Tier Estimate Worksheet Candidate Vaccines Operation Warp Speed Vaccine Safety
Tier Estimate Worksheet August 3rd deadline For those still needing assistance, please call me at 573-526-6643 For Missouri National Guard or military personnel, please only count those that are permanently stationed in your county. Please email the completed Tier Estimate worksheet to either Jennifer.VanBooven@health.mo.gov or Lana.Hudanick@health.mo.gov
Vaccine Update: Moderna Moderna s COVID 19 vaccine Scheduled to start phase 3 trials July 27th(today) mRNA based (this is new technology for vaccines) 30,000 individuals enrolled in phase 3 trials Early data is showing that it produces antibodies to neutralize the virus Mild to moderate side effects 2 injections separated by 4 weeks https://www.statnews.com/feature/coronavirus/drugs-vaccines-tracker/
Vaccine Update: AstraZeneca & University of Oxford AZD1222 COVID-19 vaccine Presently in Phase 3 trials 2 dose series Uses a modified genetically engineered virus (new platform) Has been used in experimental vaccine development for a Ebola vaccine Side effects are mild to moderate Immune response is good Only individuals 18-55 were enrolled in the trials https://www.statnews.com/feature/coronavirus/drugs-vaccines-tracker/
Vaccine Update: BioNTech and Pfizer Phase 2b/3 late July/early August Depending on successful studies, may request EUA as early as October 2020 (subject to change) 4 different versions are in the trial at this time 1-2 will move forward BNT162 Program BNT162b1 most advanced, showed the ability to produce neutralizing antibodies in humans at or above levels observed in the plasma from patients who had recovered from COVID-19 Could be 1-3 doses in a series (still evaluating) mRNA vaccine platform https://www.businesswire.com/news/home/20200722005438/en/Pfizer-BioNTech-Announce- Agreement-U.S.-Government-600 https://www.statnews.com/feature/coronavirus/drugs-vaccines-tracker/
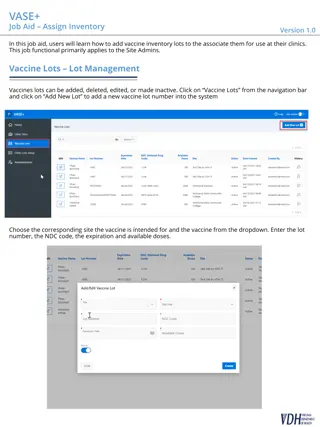
Vaccine Update Any vaccine that is available in the Fall will be Limited amounts Targeted vaccine efforts will occur Still unsure on how vaccine will be initially deployed BI has been instructed that we will need to use the ShowMeVax Missouri s Immunization Information System (IIS) to track doses once general population vaccination begins Still waiting to see who will be prioritized Advisory Committee on Immunization Practices (ACIP) meets mid August and September before regularly scheduled meeting in October
Operation Warp Speed Key players: CDC, HHS, FDA, NIH, DoD Aimed at accelerating the production of COVID-19 vaccine(s) while maintaining proper safety and efficacy measures Developing details around vaccine distribution and priority groups Closed Door meeting on Wednesday, July 22nd Hopeful some decisions were made regarding priority groups and vaccine distribution.
Vaccine Safety All adverse events that occur will need to be reported online at VAERS CDC and HHS are in the process of improving the system to handle the vaccine Once vaccine is deployed: VAERS will start collecting reports of adverse events for COVID-19 vaccine All health care providers will need to report online at https://vaers.hhs.gov/ Each Monday Missouri s Vaccine Safety Coordinator will be given an updated spreadsheet of reported events for Missouri Redacted data will be made available on CDC WONDER
Vaccine Safety How will the state be determined for each COVID-19 VAERS report? Patient state of resident is always first Where the individual was vaccinated Where the VAERS report was made How will deaths following vaccination be reported? Should a death occur then the Vaccine Safety Coordinator will receive an email
What steps should be take now Ensure all staff who administer immunizations know how to report to VAERS Any communication with immunization providers in the county should include reporting vaccine adverse events in VAERS If providing any education to an immunizer, make sure they know how to report to VAERS If you need help or education on VAERS please contact the Bureau of Immunizations
Other information Guidance will be sent out to all of your VFC coordinators on Planning Vaccination Clinics Held at Satellite, Temporary or Off-Site Locations The guidance is broken down into four categories: Planning activities (https://www.cdc.gov/vaccines/hcp/admin/mass-clinic- activities/planning-activities.html) Pre-clinic activities (https://www.cdc.gov/vaccines/hcp/admin/mass-clinic- activities/pre-clinic-activities.html) During the clinic activities (https://www.cdc.gov/vaccines/hcp/admin/mass- clinic-activities/during-clinic-activities.html) Post-clinic activities (https://www.cdc.gov/vaccines/hcp/admin/mass-clinic- activities/post-clinic-activities.html)
Next Steps Next Call August 31st3:30-4:30 Any updates between now and then I will try to provide in an email but may need to schedule an earlier call. Still working to secure PPE and vaccine administration supplies for distribution to LPHAs and other community partners engaged in vaccination efforts. Any questions?