Crystalline Solids: Structure, Metallic Crystal Types, and Allotropy
Delve into the world of crystalline solids, exploring their atomic arrangements and different crystal structures like FCC, BCC, and HCP. Learn about Atomic Packing Number, polymorphism, allotropy, and crystal systems with detailed examples in this informative guide.
Uploaded on | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
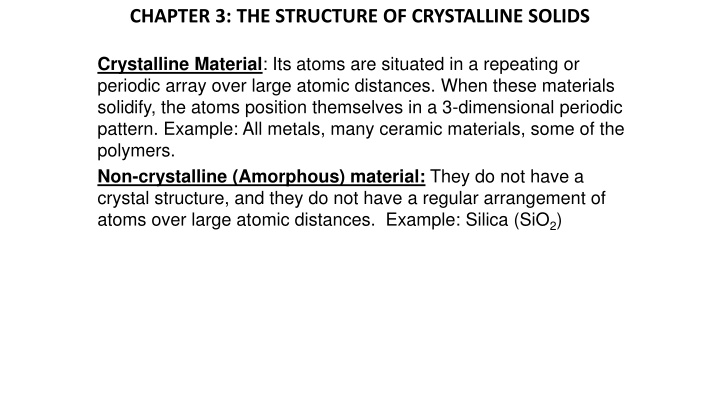
CHAPTER 3: THE STRUCTURE OF CRYSTALLINE SOLIDS Crystalline Material: Its atoms are situated in a repeating or periodic array over large atomic distances. When these materials solidify, the atoms position themselves in a 3-dimensional periodic pattern. Example: All metals, many ceramic materials, some of the polymers. Non-crystalline (Amorphous) material: They do not have a crystal structure, and they do not have a regular arrangement of atoms over large atomic distances. Example: Silica (SiO2)
CHAPTER 3: THE STRUCTURE OF CRYSTALLINE SOLIDS Metallic crystal structures: There are three types of metallic structures: 1. The face-centered cubic (FCC) crystal structure: Atoms are located at the corners of the cube and at the centers of each face of the cube. Figure 3.1b. Example: Copper, Aluminium, Silver, Gold.
Atomic Packing Number (APF): It is the ratio of volume of atoms in a unit cell to the total unit cell volume. volume of atoms in a unit cell APF = total unit cell volume
2. The Body-Centered Cubic Crystal Structure (BCC): It is a cubic unit cell with atoms located at all eight corners and a single atom at the cube center. Figure 3.2b. Cr, -Fe, Mo have this crystal structure
3. The Hexagonal Close-Packed Crystal Structure (HCP): It is a Hexagonal unit cell. The top and bottom faces of the unit cell consist of six atoms that form regular hexagons, and there is one atom at the center of each hexagon. There are also three additional atoms between the top and bottom faces of HCP. Figure 3.3a. Example: Cadmium, Magnesium, Zinc.
Polymorphism and Allotropy: Some metals and nonmetals, may have more than one crystal structure. This is called polymorphism. In solids, it is called allotropy. Example: Pure iron has a BCC structure at room temperature, but it changes to FCC structure at 912 C.
CRYSTAL SYSTEMS: Crystal systems can be divided into repeating units which are called unit cells. A unit cell has x,y,z coordinate axes, axial lengths of a,b,c and interaxial angles of , , . Figure 3.4. a,b,c and , , are called lattice parameters.
CRYSTAL SYSTEMS: There are seven crystal systems: cubic, tetragonal, hexagonal, orthorombic, rhombohedral, monoclinic, triclinic. Table. 3.2
CRYSTAL SYSTEMS: Crystallographic Direction: It is defined as a line between two points, or a vector. [xyz] Figure 3.5
CRYSTAL SYSTEMS: Crystallographic Planes: Crystallographic planes are defined by three Miller Indices as (hkl). Example: (111) plane. CRYSTALLINE AND NONCRYSTALLINE MATERIALS Single Crystals: If the arrangement of atoms in a crystalline solid is perfect or extends without interruption, the crystalline solid is called a single crystal. Single crystals exist in nature, they can be also made in the lab only with a controlled environment. They are used in electronic microcircuits.
Polycrystalline Materials: Most crystalline solids are composed of a collection of many small crystals or grains; such materials are called polycrystalline. The area between two grains in a polycrystalline material is called a grain boundary. Solidification process of a polycrystalline material. Figure 3.18.
