Custom Workflow for Drug Substance Development in Pre-term Labour Treatment
"Explore the sequential workflow designed by Kate Llewellyn at GlaxoSmithKline for developing a drug substance to treat pre-term labor. The process involves in-depth analysis, experimental design, and identification of critical process parameters. Discover how custom designs and bespoke analyses optimize chemical reactions, crystallization, and impurity purging for successful Phase III clinical trials."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
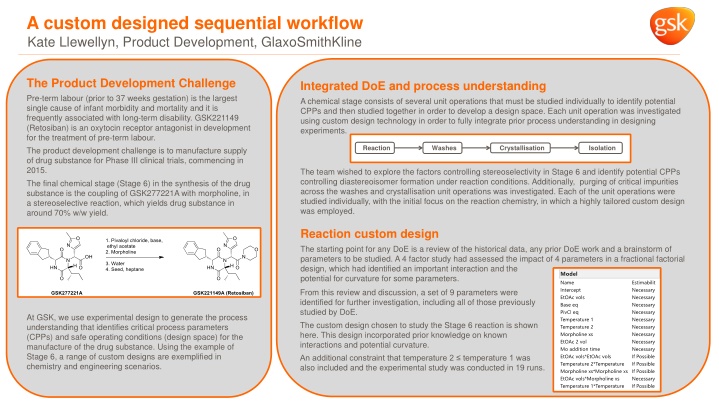
A custom designed sequential workflow Kate Llewellyn, Product Development, GlaxoSmithKline The Product Development Challenge Pre-term labour (prior to 37 weeks gestation) is the largest single cause of infant morbidity and mortality and it is frequently associated with long-term disability. GSK221149 (Retosiban) is an oxytocin receptor antagonist in development for the treatment of pre-term labour. The product development challenge is to manufacture supply of drug substance for Phase III clinical trials, commencing in 2015. The final chemical stage (Stage 6) in the synthesis of the drug substance is the coupling of GSK277221A with morpholine, in a stereoselective reaction, which yields drug substance in around 70% w/w yield. Integrated DoE and process understanding A chemical stage consists of several unit operations that must be studied individually to identify potential CPPs and then studied together in order to develop a design space. Each unit operation was investigated using custom design technology in order to fully integrate prior process understanding in designing experiments. Reaction Washes Crystallisation Isolation The team wished to explore the factors controlling stereoselectivity in Stage 6 and identify potential CPPs controlling diastereoisomer formation under reaction conditions. Additionally, purging of critical impurities across the washes and crystallisation unit operations was investigated. Each of the unit operations were studied individually, with the initial focus on the reaction chemistry, in which a highly tailored custom design was employed. Reaction custom design The starting point for any DoE is a review of the historical data, any prior DoE work and a brainstorm of parameters to be studied. A 4 factor study had assessed the impact of 4 parameters in a fractional factorial design, which had identified an important interaction and the potential for curvature for some parameters. From this review and discussion, a set of 9 parameters were identified for further investigation, including all of those previously studied by DoE. The custom design chosen to study the Stage 6 reaction is shown here. This design incorporated prior knowledge on known interactions and potential curvature. An additional constraint that temperature 2 temperature 1 was also included and the experimental study was conducted in 19 runs. At GSK, we use experimental design to generate the process understanding that identifies critical process parameters (CPPs) and safe operating conditions (design space) for the manufacture of the drug substance. Using the example of Stage 6, a range of custom designs are exemplified in chemistry and engineering scenarios.
A custom designed sequential workflow Kate Llewellyn, Product Development, GlaxoSmithKline Custom design analysis The data from this design was then analysed using several platforms in JMP11. In most cases, simple models may be identified using the screening platform. Crystallisation Bespoke designs to solve chemistry problems The remaining unit operations, the washing and crystallisation were studied in smaller scale bespoke designs, intended to quickly identify any interactions across the unit operations and provide assurance of robustness prior to scale-up. In order to meet the deadline for scale-up of the chemistry, a fractional factorial design was initially selected. The two most forcing sets of conditions in the design were run first. During processing, issues with performance of the crystallisation were observed. A change to ranges was needed to avoid problematic conditions, producing an unbalanced design which was also analysed in JMP. However in some case, the screening platform fails to identify a sensible model. In these cases, multiple alternative models can be assessed using all possible models within the Stepwise platform. Washing A custom design was targeted at understanding the impact of water in washes on purging of key impurities in this unit operation. The model was specified so that the main effect of water, the crossed term for water and a key interaction of water with input were not aliased. Models across multiple potentially correlated responses are visualised using the PCA platform. Impurities that are formed by similar mechanisms can be identified, grouped and remodelled. Outcomes & next steps Following the completion of the sequence of experiments across the three unit operations, the manufacture of Phase III clinical supplies was conducted in Singapore. Six batches at 2.9 kg each were manufactured in excellent yield and quality. Next steps for Stage 6 will include revisiting the problematic crystallisation and exploring how the established ranges perform as the specification of the input materials vary. At each stage, we will be custom designing the experiments to solve the unique chemistry problems. Selection of processing set points and ranges, is enabled by visualising multiple responses using the prediction profiler. The prediction profiler is used throughout to visualise the size and direction of parameter effects.
Reaction custom design analysis Back 2 Back 1 Back 2 Back 1 Combining all data in a PCA model Defining the model Rapid analysis in the screening platform Modelling of key responses Exploring alternative models in all possible models
Workup custom design analysis Back Back Assess colinearity using VIFs Defining the model Resolving conflicts through visualising multiple responses Visualising column correlations
Crystallisation edited fractional factorial Back Back Inputting the original model Visualising correlations when things go wrong! Visualising model outputs