Daily Routine & Management in Dairy Farming: Pregnancy Care & Steaming Up
This informative content covers the daily operations in dairy farms, including managing pregnant cows, diagnosing pregnancy, approaching parturition, and steaming up for optimal calf development and lactation. Learn about the key practices, tools, and techniques involved in dairy farming to ensure the health and productivity of your herd.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
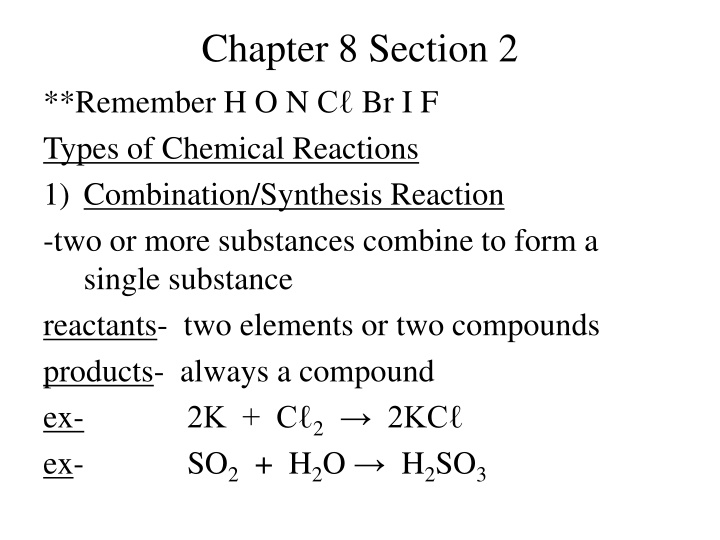
Chapter 8 Section 2 **Remember H O N C Br I F Types of Chemical Reactions 1) Combination/Synthesis Reaction -two or more substances combine to form a single substance reactants- two elements or two compounds products- always a compound ex- 2K + C 2 2KC ex- SO2+ H2O H2SO3
Try these!! Dont forget to balance!! a) aluminum + oxygen A + O2 A + O2 A 2O3 b) beryllium + oxygen Be + O2 Be + O2 BeO c) strontium + iodine Sr + I2 Sr + I2 SrI2 d) magnesium + nitrogen Mg + N2 Mg + N2 Mg3N2
2) Decomposition Reaction -one compound breaks down or decomposes into two simpler compounds -the reverse of synthesis ex- 2H2O 2H2+ O2 Try these!! a) lead(IV) oxide PbO2 PbO2 Pb + O2 b) hydrogen iodide HI HI H2+ I2
c) hydrogen bromide HBr HBr H2+ Br2 d) sodium chloride NaC NaC Na + C 2
3) Single-Replacement Reactions -one element replaces a second element in a compound ex- 2K + CaO K2O + Ca -whether one metal will replace another metal is determined by reactivity of the metal activity series of metals- lists metals in order of decreasing reactivity **Page 217 -a metal will replace another metal listed below it in the table
ex- Mg + Zn(NO3)2 *is Mg above Zn on the reactivity series? Mg + Zn(NO3)2 Mg(NO3)2+ Zn ex- Mg + Ag2SO4 Mg + Ag2SO4 MgSO4 + Ag ex- Mg + LiNO3 -lithium is above magnesium Mg + LiNO3 no reaction (NR)
-Halogens can replace each other in single- replacement reactions -Reactivity decreases as you go down the halogen group Try These!! a) zinc + hydrogen sulfate Zn + H2SO4 Zn + H2SO4 H2+ ZnSO4 b) chlorine + sodium bromide C 2+ NaBr C 2+ NaBr Br2+ NaC
c) zinc + sodium nitrate Zn + NaNO3 no reaction d) iron(II) + lead(II) nitrate Fe + Pb(NO3)2 Fe + Pb(NO3)2 Pb + Fe(NO3)2 e) chlorine + sodium iodide C 2+ NaI C 2+ NaI I2+ NaC
4) Double-Replacement Reactions -involve an exchange of cations between two reacting compounds ex- BaC 2+ K2CO3 BaCO3+ 2KC ex- FeS + 2HC H2S + FeC 2 Try These!! a) sodium hydroxide + iron(III) nitrate NaOH + Fe(NO3)3 NaOH + Fe(NO3)3 NaNO3+ Fe(OH)3
b) barium nitrate + hydrogen phosphate Ba(NO3)2+ H3PO4 Ba(NO3)2+ H3PO4 Ba3(PO4)2+ HNO3 c) potassium hydroxide+hydrogen phosphate KOH + H3PO4 KOH + H3PO4 K3PO4 + H2O d) hydrogen sulfate + aluminum hydroxide H2SO4 + A (OH)3 H2SO4 + A (OH)3 A 2(SO4)3+ H2O
5) Combustion Reaction -hydrocarbon combined with oxygen to produce carbon dioxide and water ex- C6H6+ O2 CO2+ H2O -to balance: *always begin with a 2 in front of hydrocarbon *when done, if can divide by 2 do so 2C6H6+ 15O2 12CO2+ 6H2O
Try These!! a) C14H26+ O2 2C14H26+ 41O2 28CO2+ 26H2O b) C8H12+ O2 2C8H12+ 22O2 16CO2+ 12H2O C8H12+ 11O2 8CO2+ 6H2O
Summary of Reactions 1) Combination/Synthesis R + S RS 2) Decomposition Reaction RS R + S 3) Single-Replacement Reaction T + RS R + TS 4) Double-Replacement Reaction RS + TU RU + TS 5) Combustion Reaction CxHy+ O2 CO2+ H2O