Demonstrations of Condensation & Dew Point
Fascinating phenomena of condensation and dew point through engaging demonstrations. Witness condensation forming on both the outside and inside surfaces of bottles, understand the science behind these occurrences, and learn about evaporation effects. Discover the practical implications of these concepts, such as the Rainshadow effect and adiabatic heating. This insightful project sheds light on water vapor transformations in different environmental conditions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
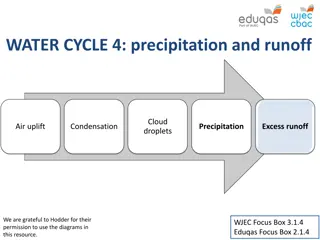
Lesson 1 Demonstrations Condensation and DewPoint
Condensation on the Outside O If you live in a sufficiently humid climate (won t work in the desert), keep a bottle of water in the refrigeratorovernight. O Pull it out and watch for condensation to occur onthe outside surface. O Ask the students, where did that water come from? Was it from the inside? What will happen to this condensation as it warms up to room temperature and why? You can also take a bottleout of the freezer and watch frost form on the outside surface. Ask themwhy that occurred.
Condensation on the Inside O Take an empty jar or bottle out of the refrigerator and let sit for 3minutes. O While waiting, heat a small quantity of water above 100 F. O Pour little hot water into thejar. O Condensation will form on theinside. O Ask the students why this isoccurred. Hot water added to cooljar
Evaporation on the Inside O Place the bottle in the refrigerator for a few minutes and check back on it (this represents cooler air in themountains). O You will notice that the condensation is even more apparent. Ask the students why. O Take it out of the refrigerator and pour hot water over the outside of the jar. You will see the condensation on the inside will evaporate where it was heated by the water. O Explain that this is the equivalent to the Rainshadow effect and the clouds evaporating, due to adiabaticheating. Line where warm water pouring on outside evaporated condensation on inside
This project was made possible in part by a grant from Washington s National Park Fund.