DENSITY AND TEMPERATURE
Discover the fundamentals of density and temperature in this educational journey. Learn about density, its calculation, significance, and its relation to mass and volume. Explore the concept of temperature, scales of measurement, and the properties of water. Engage in sample problems to enhance your understanding and practice calculating density.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
DENSITY AND TEMPERATURE
What is Density?? What is Density??
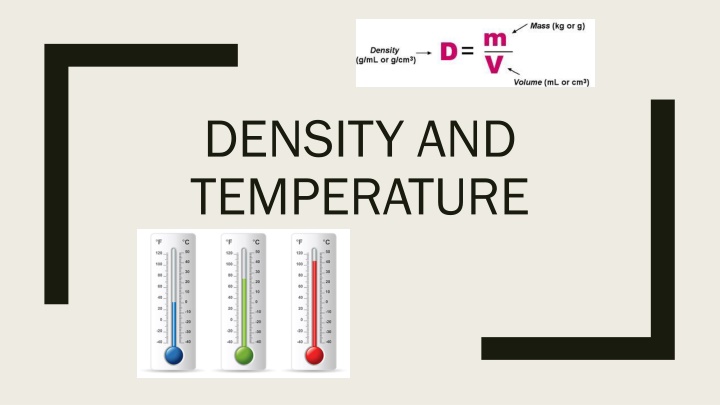
What is density? What is density? Density is mass per unit volume of a substance Ice floats in water because Ice is less dense than water Liquids with low densities will float on top of substances that are more dense Density= Mass/Volume Units: Mass grams (g) Volume milliliters (mL) or Cubic centimeters (cm3)
Two Ways to Volume Two Ways to Volume Volumes of Solids Volumes of Solids We use the formula specific to their shape Cylinders = x r2 x h Blocks = l x w x h Volumes of liquids Volumes of liquids We use water displacement
Sample Problem 1 Sample Problem 1 Calculate the density of a material that has a mass of 52.457 g and a Calculate the density of a material that has a mass of 52.457 g and a volume of 13.5 cm volume of 13.5 cm3 3. . D = ??? M = 52.457 g V = 13.5 cm3 D = 52.457/13.5 D= 3.8857 3.89g/cm 3.89g/cm3 3
Sample Problem 2 Sample Problem 2 The density of silver is 10.49 g/cm The density of silver is 10.49 g/cm3 3. . If a sample of pure silver has a volume of 12.993 cm volume of 12.993 cm3 3, what is the mass? , what is the mass? D = 10.49 g/cm3 M = ??? V = 12.993 cm3 10.49 = M /12.993 M = D x V 136.296 136.3 g If a sample of pure silver has a 136.3 g
Practice on Your Own A student finds a rock on the way to school. In the laboratory he determines that the volume of the rock is 22.7 mL, and the mass in 39.943 g. What is the density of the rock? What is the mass of a 350 cm3sample of pure silicon with a density of 2.336 g/cm3? The density of lead is 11.342 g/mL. What would be the volume of a 200.0 g sample of this metal?
Temperature: Temperature: The measurement of the AVERAGE KINETIC energy of a particle. Scientists use two equivalent scales: Celsius and the Kelvin. Absolute Zero: Absolute Zero: Theoretically the point at which all particle motion ceases. Zero Kelvin For WATER: For WATER: On the Celsius scale: On the Celsius scale: The Freezing point is ZERO the boiling point is 100. On the Kelvin scale: On the Kelvin scale: The freezing point is 273.15 and the boiling point is 373.15
Temperature Conversions Celsius To Kelvin Kelvin to Celsius Formula: K = C + 273 Formula: C = K 273 EX: Covert 23 C to K K = 23 + 273 = 296 K EX: Convert 400K to Celsius C = 400-273 = 127
Practice Convert the Celsius temperatures to Kelvin: -50 C 100 C 32 C Convert the Kelvin temperatures to Celsius: 150K 300K 125 K 25 K