Density of States and Bloch's Theorem
The density of states in materials can be determined using mathematical approaches involving length, area, and volume. Bloch's Theorem and the Krönig-Penney model help relate wavefunctions and energy levels in quantum systems.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
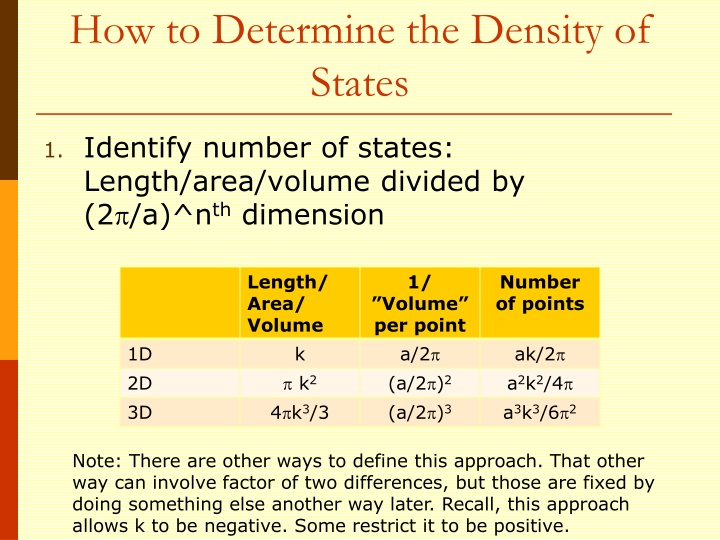
How to Determine the Density of States 1. Identify number of states: Length/area/volume divided by (2 /a)^nthdimension Length/ Area/ Volume 1/ Number of points Volume per point a/2 (a/2 )2 (a/2 )3 ak/2 a2k2/4 a3k3/6 2 1D 2D 3D k k2 4 k3/3 Note: There are other ways to define this approach. That other way can involve factor of two differences, but those are fixed by doing something else another way later. Recall, this approach allows k to be negative. Some restrict it to be positive.
How to Determine the Density of States 1. Identify number of states: Length/area/volume divided by (2 /a)^nthdimension 2. The density of levels is the dN/dk 3. The density of states is the density of levels times any degeneracy. Gives D(k) 4. D(k) dk = D(E) dE, so D(E) = D(k) dE/dk Check to make sure your D(E) no longer has any k s in it.
= + iKx iKx ( ) x Ae Be I Boundary Conditions and Bloch s Theorem The solutions of the SE require that the wavefunction and its derivative be continuous across the potential boundaries. Thus, at the two boundaries (which are infinitely repeated): C B A = + x x = + ( ) x Ce De II = ( ) ( ) iK A B C D (2) + D (1) x = 0 Use Bloch s Theorem otherwise wavefunction not in terms of k.
= + iKx iKx ( ) x Ae Be I Boundary Conditions and Bloch s Theorem The solutions of the SE require that the wavefunction and its derivative be continuous across the potential boundaries. Thus, at the two boundaries (which are infinitely repeated): C B A = + x x = + ( ) x Ce De II + = = ( ) ( ) iK A B C D (2) + D (1) x = 0 ikR ( ) ( ) x R e x + = iKa iKa (a ) Ae Be x = a II Now using Bloch s theorem for R= -(a+b): + = ( ) ik a b ( ) ( ) a b e k = Bloch wavevector II II Now we can write the boundary conditions at x = a: The four simultaneous equations (1-4) can be written compactly in matrix form Let s start it! (3) + + = + ( ) iKa iKa b b ik a b ( ) Ae Be Ce De e + + = + ( ) iKa iKa b b ik a b (4) ( ) ( ) ( ( ) ( ) ) iK ik Ae iK ik Be ik Ce ik De e
Results of the Krnig-Penney Model 1 1 iK 1 1 A e iK B = 0 + + ( ) ( ) iKa iKa b ik a b b ik a b e e e e e C + + + + + ( ) ( ) iKa iKa b ik a b b ik a b ( ) ( ) ( ) ( ) iK ik e iK ik e ik e e ik e e D + = + A B C = D (1) ( ) ( ) iK A B C D (2) (3) + + = + ( ) iKa iKa b b ik a b ( ) Ae Be Ce De e + + = + ( ) iKa iKa b b ik a b (4) ( ) ( ) ( ( ) ( ) ) iK ik Ae iK ik Be ik Ce ik De e
Results of the Krnig-Penney Model 1 1 iK 1 1 A e iK B = 0 + + ( ) ( ) iKa iKa b ik a b b ik a b e e e e e C + + + + + ( ) ( ) iKa iKa b ik a b b ik a b ( ) ( ) ( ) ( ) iK ik e iK ik e ik e e ik e e D Since the values of a and b are inputs to the model, and depends on U0and the energy E, we can solve this system of equations to find the energy E at any specified value of the Bloch wavevector k. What is the easiest way to do this? Taking the determinant, setting it equal to zero and lots of algebra gives: 2 2 K ) b ( ) ( ) b ( ) ( ) b + = + sin sinh cos cosh cos ( Ka Ka k a 2 K By reducing the barrier width b (small b), this can be simplified to:
Graphical Approach 2 2 K ) b ( ) ( ) b ( ) ( ) b + = + sin sinh cos cosh cos ( Ka Ka k a 2 K small b 2 b ( ) ( ) + = sin cos cos( ) Ka Ka ka 2 K Right hand side cannot exceed 1, so values exceeding will mean that there is no wavelike solutions of the Schrodinger eq. (forbidden band gap) 2 2 Problems occur at Ka=N or K=N /a K = Eregion 1 2 m Plotting left hand side Ka
Turning the last graph on its side 2 b ( ) ( ) + = sin cos cos( ) Ka Ka ka 2 K This equation determines the energy bands. BAND 2 E(k) (a.u.) For values of K where the left side of the equation has a magnitude < 1, then k is real and energy bands are allowed. Forbidden band gap Ka/ BAND 1 2 2 = E 0 2 2ma 2 b 2 ( ) ( ) ka/ + sin cos Ka Ka K
To better understand this, lets compare to the free-electron model Free electron dispersion E 2 = + + 2 2 2 ( ) E k k k x y z 2 m k /a /a Let s slowly turn on the periodic potential
E Electron Wavefunctions in a Periodic Potential (Another way to understand the energy gap) Consider the following cases: Wavefunctions are plane waves and energy bands are parabolic: = ( ) i kx t Ae = 1= 0 U 2 2 k k /a /a E 2 m U U1 x -b 0 aa+b 2a+b 2(a+b)
E Electron Wavefunctions in a Periodic Potential (Another way to understand the energy gap) Consider the following cases: Wavefunctions are plane waves and energy bands are parabolic: = ( ) i kx t Ae = 1= 0 U 2 2 k k /a /a E 2 m 0 U Electrons wavelengths much larger than atomic spacing a, so wavefunctions and energy bands are nearly the same as above 1 k a U U1 x -b 0 aa+b 2a+b 2(a+b)
Wavelength much greater than atomic spacing Energy of wave Similar to how radio waves pass through us without affecting
Wavelength much greater than atomic spacing Energy of wave What happens as I lower this energy? Similar to how radio waves pass through us without affecting
E Electron Wavefunctions in a Periodic Potential U=barrier potential Consider the following cases: Wavefunctions are plane waves and energy bands are parabolic: = ( ) i kx t Ae = 1= 0 U 2 2 k k /a /a E 2 m 0 U Electrons wavelengths much larger than a, so wavefunctions and energy bands are nearly the same as above Electrons wavelengths approach a, so waves begin to be strongly back-scattered by the potential: ) ( t kx i Be Ae = 1 k a 0 U 1 k a B ( ) i kx t A
E Electron Wavefunctions in a Periodic Potential U=barrier potential Consider the following cases: Wavefunctions are plane waves and energy bands are parabolic: = ( ) i kx t Ae = 1= 0 U 2 2 k k /a /a E 2 m 0 U Electrons wavelengths much larger than a, so wavefunctions and energy bands are nearly the same as above Electrons wavelengths approach a, so waves begin to be strongly back-scattered by the potential: ) ( t kx i Be Ae = 1 k a 0 U 1 k a B ( ) i kx t A 0 U Electrons waves are strongly back-scattered (Bragg scattering) so standing waves are formed: e A e e = 2 1 = k a = ( ) ( ) i kx t i kx t ikx ikx i t C e e 1
The nearly-free-electron model (Standing Waves) Due to the , there are two such standing waves possible: = ikx ikx i t A e e e 1 2
The nearly-free-electron model (Standing Waves) Due to the , there are two such standing waves possible: A e e e A + = + = cos( 2 2 2 iA e e e A = = sin( 2 2 2 ikx ikx i t i t ) kx e 1 1 = ikx ikx i t A e e e 1 2 ikx ikx i t i t ) kx e 1 1
The nearly-free-electron model (Standing Waves) Due to the , there are two such standing waves possible: A e e e A + = + = cos( 2 2 2 iA e e e A = = sin( 2 2 2 These two approximate solutions to the S. E. at have very different potential energies. has its peaks at x = a, 2a, 3a, at the positions of the atoms, where V is at its minimum (low energy wavefunction). The other solution, has its peaks at x = a/2, 3a/2, 5a/2, at positions in between atoms, where V is at its maximum (high energy wavefunction). ikx ikx i t i t ) kx e 1 1 = ikx ikx i t A e e e 1 2 ikx ikx i t i t ) kx e 1 1 = k a + Either: Nodes at ions Nodes midway between ions Or: a
The nearly-free-electron model ~ 2 Strictly speaking we should have looked at the probabilities before coming to this conclusion: * + = = + = 2 2 ikx ikx i t i t 2 cos ( ) 2 cos( ) A A e e e A kx e x 1 1 + + a 2 2 2 = * = = 2 2 ikx ikx i t i t sin ( ) 2 sin( ) A A e e e iA kx e x 1 1 a 2 2 2 Different energies for electron standing waves 2 a
Summary: The nearly-free-electron model BAND GAPS APPEAR AT EACH BRILLOUIN ZONE EDGE E The periodic potential V(x) splits the free- electron E(k) into energy bands separated by gaps at each BZ boundary. In between the two energies there are no allowed energies; i.e., wavelike solutions of the Schrodinger equation do not exist. E- Eg E+ 20 k -2 /a /a /a 2 /a Forbidden energy bands form called band gaps.
Approximating the Band Gap BAND GAPS APPEAR AT EACH BRILLOUIN ZONE EDGE a E 2 2 = + = = ( )[ dx ] E E E U x + g 0 x E- Eg E+ k -2 /a /a /a 2 /a
Approximating the Band Gap BAND GAPS APPEAR AT EACH BRILLOUIN ZONE EDGE a E 2 2 = + = = ( )[ dx ] E E E U x + g 0 x * + = 2 2 2 cos ( ) A x + a = * 2 2 2 sin ( ) A x a E- Eg E+ k -2 /a /a /a 2 /a
Approximating the Band Gap BAND GAPS APPEAR AT EACH BRILLOUIN ZONE EDGE a E 2 2 = + = = ( )[ dx ] E E E U x + g 0 x * + = 2 2 2 cos ( ) A x + a = * 2 2 2 sin ( ) A x a E- Eg a E+ = ( ) cos ( ) E E U x dx 2 x 2 + a a k 0 x -2 /a /a /a 2 /a
Approximating the Band Gap BAND GAPS APPEAR AT EACH BRILLOUIN ZONE EDGE a E 2 2 = + = = ( )[ dx ] E E E U x + g 0 x * + = 2 2 2 cos ( ) A x + a = * 2 2 2 sin ( ) A x a E- Eg a E+ = ( ) cos ( ) E E U x dx 2 x 2 + a a k 0 x -2 /a /a /a 2 /a For square potential: V(x) =Vofor specific values of x (changes integration limits)
Just need Fourier components of U ?????? ? ? = ? Values of UGdecrease rapidly with increasing G. 2 2? ?2? ? ??2 + ? ? ? ? = ?? ? By pulling into Sch. Equ, it can be shown that the wavefunction may be expressed as a Fourier series summed over all values of the wavevector ? = ?? ? ????
Two Common Approaches Do Non-Physicists Think Quantum Wells are Easy? A Physicist Thinks Quantum Wells are Easy (Kroniq-Penney Model or Nearly Free Electron Approx.) Chemist s View - Start with atomic energy levels & build up the periodic solid by decreasing distance between atoms (tightbinding or linear combination of atomic orbitals U(x) x d
Approach 2: Tightbinding or Linear Combination of Atomic Orbitals (LCAO) Assume the atomic orbitals ~ unchanged
Approach 2: Tightbinding or Linear Combination of Atomic Orbitals (LCAO) Assume the atomic orbitals ~ unchanged bare atoms solid Atomic energy levels merge to form molecular levels & merge to form bands
Compare to hydrogen When atoms are covalently bonded electrons are shared by atoms
Compare to hydrogen When atoms are covalently bonded electrons are shared by atoms Example: the ground state of the hydrogen atoms forming a molecule If atoms far apart, little overlap
Compare to hydrogen When atoms are covalently bonded electrons are shared by atoms Example: the ground state of the hydrogen atoms forming a molecule If atoms far apart, little overlap If atoms are brought together the wavefunctions overlap and form the compound wavefunction, 1(r)+ 2(r), increasing the probability for electrons to exist between atoms
Compare to hydrogen When atoms are covalently bonded electrons are shared by atoms Example: the ground state of the hydrogen atoms forming a molecule If atoms far apart, little overlap If atoms are brought together the wavefunctions overlap and form the compound wavefunction, 1(r)+ 2(r), increasing the probability for electrons to exist between atoms 2 2 d ( x ) 2 , 1 dx + = U ( x )[ ( x )] E ( x ) 2 , 1 2 , 1 2 2 x m + [ 2 2 d ( ) ( x )] + + = [ + 1 2 U ( x )[ ( x ) ( x )] E ( x ) ( x )] 1 2 1 2 2 2 m dx
Compare to hydrogen When atoms are covalently bonded electrons are shared by atoms Example: the ground state of the hydrogen atoms forming a molecule If atoms far apart, little overlap If atoms are brought together the wavefunctions overlap and form the compound wavefunction, 1(r)+ 2(r), increasing the probability for electrons to exist between atoms These two possible combinations represent 2 possible states of two atoms system with different energies 2 2 d ( x ) 2 , 1 dx + = U ( x )[ ( x )] E ( x ) 2 , 1 2 , 1 2 2 x m + [ 2 2 d ( ) ( x )] + + = [ + 1 2 U ( x )[ ( x ) ( x )] E ( x ) ( x )] 1 2 1 2 2 2 m dx
LCAO: Electron in Hydrogen Atom (in Ground State) Ae = r K x ( ) 1 1 2.0 1.5 1(r) 1.0 0.5 0.0 -10 -5 0 5 10 r (aB)
LCAO: Electron in Hydrogen Atom (in Ground State) Ae = r K x ( ) 1 1 Second hydrogen atom ) ( 2 = r Ae K x 2 2.0 1.5 1(r) 1.0 0.5 0.0 -10 -5 0 5 10 r (aB)
LCAO: Electron in Hydrogen Atom (in Ground State) Ae = r K x ( ) 1 1 Second hydrogen atom ) ( 2 = r Ae K x 2 Do you see a pattern? 2.0 1.5 1(r) 1.0 0.5 0.0 -10 -5 0 5 10 r (aB) Number of Nodes?
LCAO: Electron in Hydrogen Atom (in Ground State) Ae = r 5 K x ( ) 1 4 1 Second hydrogen atom ) ( 2 = r 3 Ae K x 2 2 Do you see a pattern? 2.0 1 1.5 1(r) 1.0 0 0.5 0.0 -10 -5 0 5 10 r (aB) Number of Nodes?
LCAO: Electron in Hydrogen Atom (in Ground State) Ae = r 5 K x ( ) 1 4 1 Second hydrogen atom ) ( 2 = r 3 Ae K x 2 2 Do you see a pattern? 2.0 1 1.5 1(r) 1.0 0 0.5 0.0 -10 -5 0 5 10 r (aB) Number of Nodes?
If there are N atoms in the chain there will be N energy levels and N electronic states (molecular orbits). The wavefunction for each electronic state is: k= eikna n= k= /a n k= /2a a is the lattice constant, n identifies the individual atoms within the chain, nrepresents the atomic orbitals of each individual atom k=0 a
If there are N atoms in the chain there will be N energy levels and N electronic states (molecular orbits). The wavefunction for each electronic state is: k= eikna n= e0 0+eika 1+e2ika 2+e3ika 3+e4ika 4 k= /a n k= /2a a is the lattice constant, n identifies the individual atoms within the chain, nrepresents the atomic orbitals of each individual atom k=0 a
If there are N atoms in the chain there will be N energy levels and N electronic states (molecular orbits). The wavefunction for each electronic state is: k= eikna n= e0 0+eika 1+e2ika 2+e3ika 3+e4ika 4 k= /a n k= /2a a is the lattice constant, n identifies the individual atoms within the chain, nrepresents the atomic orbitals of each individual atom k is a quantum # that identifies the wavefunction and tells us the phase of the orbitals. k=0 a The larger the absolute value of k, the more nodes one has
Group: For 0, 2 and 4 nodes, determine wavefunctions (plug in n and k) If there are N atoms in the chain there will be N energy levels and N electronic states (molecular orbits). The wavefunction for each electronic state is: k= eikna n= e0 0+eika 1+e2ika 2+e3ika 3+e4ika 4 k= /a n k= /2a a is the lattice constant, n identifies the individual atoms within the chain, nrepresents the atomic orbitals of each individual atom k is a quantum # that identifies the wavefunction and tells us the phase of the orbitals. k=0 a The larger the absolute value of k, the more nodes one has
Infinite 1D Chain of H atoms k= /a k= /2a k = 0 k=0 0= 0+ 1 + 2 + 3 + 4 + a
Infinite 1D Chain of H atoms k = /a /a= 0+(exp{i }) 1 +(exp{i2 }) 2 +(exp{i3 }) 3+(exp{i4 }) 4+ k= /a k= /2a k = 0 k=0 0= 0+ 1 + 2 + 3 + 4 + a
Infinite 1D Chain of H atoms k = /a /a= 0+(exp{i }) 1 +(exp{i2 }) 2 +(exp{i3 }) 3+(exp{i4 }) 4+ /a= 0 - 1 + 2 - 3 + 4 + k= /a k= /2a k = 0 k=0 0= 0+ 1 + 2 + 3 + 4 + a k=0 k= /a orbital phase does not change when we translate by a orbital phase reverses when we translate by a
Infinite 1D Chain of H atoms k = /a /a= 0+(exp{i }) 1 +(exp{i2 }) 2 +(exp{i3 }) 3+(exp{i4 }) 4+ /a= 0 - 1 + 2 - 3 + 4 + k= /a k = /2a k= /2a /2a= 0+(exp{i /2}) 1 +(exp{i }) 2 +(exp{i3 /2}) 3+(exp{i2 }) 4+ /2a= 0 + i 1- 2 - i 3+ 4 + k = 0 k=0 0= 0+ 1 + 2 + 3 + 4 + a k=0 k= /a orbital phase does not change when we translate by a orbital phase reverses when we translate by a
Infinite 1D Chain of H atoms What would happen if consider k> /a? If not obvious, try k=2 /a. What is the wavefunction? k= eikna n
Energy bands of a crystal Tight binding model results in the same form of energy bands as in the nearly free electron model Note the higher energy states are nearly free This method gives good core electron bands! The high bands are not as good because electrons act free!
Effect of Orbital Overlap band width or dispersion=the difference in energy between the highest and lowest energy levels in the band Band width
Effect of Orbital Overlap band width or dispersion=the difference in energy between the highest and lowest energy levels in the band If we reduce the lattice parameter a (bring closer together) it has the following effects: