Determining Atomic Mass of Boron
Calculate the atomic mass of Boron based on the isotopic information provided for Boron-10 and Boron-11. Understand how to determine atomic mass using the abundance and mass of isotopes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
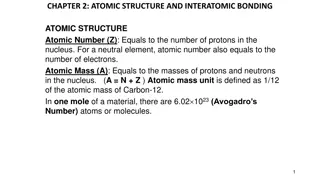
Chemistry Oct 31, 2016 P3 Challenge- Determine the atomic mass of Boron given the following information Boron 10 Mass: 10.012937 % Abundance: 19.9 Boron 11 Mass: 11.009305 % Abundance: 80.1 Get out Neutral Atoms Worksheet for Hmk check Objective Beanium Lab Activity
Chemistry Oct 24, 2016 Agenda Homework Review Beanium Lab Activity Assignment: - Beanium Lab Activity questions
Homework Review Element Oxygen Hydrogen Hydrogen Carbon Carbon Zinc Potassium Symbol O H H C C Zn K Z 8 1 1 6 6 30 19 A 16 1 3 14 12 65 39 #p 8 1 1 6 6 30 19 #n 8 0 2 8 6 35 20 #e 8 1 1 6 6 30 19
Homework Review Element Titanium Antimony Uranium Silver Fermium Platinum Krypfon Symbol Ti Sb U Ag Fm Pt Kr Z A 48 122 238 108 257 195 84 #p 22 51 92 47 100 78 36 #n 26 71 146 61 157 117 48 #e 22 51 92 47 100 78 36 22 51 92 47 100 78 36
Homework Review 1. Equal 2. A = #p+#n 3. # protons 4. (Mass)(Ab) + (Mass)(Ab) (Ab in decimal form .move decimal 2 to left) Agrees with Periodic Table Agrees with Periodic Table 6.941 5. 39.947 6. 43.38% Na 7. 52.9% Al and 47.1 % O 8. 264 g carbon dioxide
Exit Slip - Homework Exit Slip: Clean up your lab space with a zero impact from when you came in. What s Due? (Pending assignments to complete.) Beanium Lab Activity questions What s Next? (How to prepare for the next day) Read Holt p84 - 88