Developing Disease-Resistant Cassava Using CRISPR-Cas9 System
Cassava, a crucial crop for millions, faces threats like brown streak disease. The use of CRISPR-Cas9 genome editing offers a sustainable solution by developing viral-resistant cassava strains. This approach aims to combat plant diseases and enhance food security on a global scale.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
DEVELOPMENT OF DISEASE RESISTANT CASSAVA USING CRISPR-CAS9 GENOME EDITING SYSTEM Final Presentation By: Sushrutha Nagaraj Date: 8/1/2017 Class: ENSC S-135 Biochemical Engineering and Synthetic Life
CASSAVA BROWN STREAK DISEASE: A GLOBAL CRISIS Cassava is a drought-resistant and tuber-producing plant, it is a staple crop in Africa and other areas of the developing world. It is a major food source for half a billion people living in Africa, Asia, and Latin America, but has a very low yield ( about 40%) due to plant diseases. The brown streak disease virus is the most common and deadly cassava disease and it destroys cassava leaves and tubers by necrotic rotting, thus threatening the food security of the hundreds of million people who rely on this plant. This disease is caused by two distinct species of ipomoviruses belonging to the family Potyviridae.
PROPOSED SOLUTION: We propose to grow a strain of cassava with genetic resistance to potyvirus infection. We want to accomplish it through the application of the CRISPR system to combat potyvirus infections in cassava (and eventually target other viruses in different crops , this can serve as a sustainable solution to global food challenges). Target population: Cassava in some form feeds more than 750 million to a billion people each day. It the fourth largest source of calories in the world, 300 million people in Sub-Saharan Africa rely on it for food and commerce.
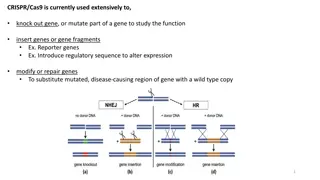
WHY CRISPR-CAS 9? Disease resistant cassava has been developed through breeding and other transgenic methods. However its not been very effective and still not commercially viable due to Anti-GMO regulations. What makes us different? We plan to use CRISPR-CAS9 editing to transfer heritable mutations (disease resistance) and we also plan to remove the CRISPR/Cas9 integrated vectors from the genome using simple genetic segregation. Thus, leaving no trace of the genome modification other than the mutation itself. Implications: According to the FDA regulations (in US, Canada and Argentina)- This variant/strain can be considered as non-GMO, since it contains gene combinations that could have been obtained through crossing or random mutation.
CRISPR-CAS9 DESIGN: A NEW APPROACH Plan to integrate a pre-assembled Cas9-gRNA ribonucleoprotein (RNP) , which is delivered to plant cells ( into the embryogenic callus) via injecting plasmids that are coding for the disease resistance gene, which can edit the desired region of the genome before being rapidly degraded by the plant s endogenous proteases and nucleases. Challenges: To create a variant of Cassava which has viral resistance and at the same time has low numbers of off-target mutations. FDA safety concerns/Regulatory approval and one viable option would be validation off- target mutations through whole genome sequencing to mitigate any safety concerns.
ETHICAL ISSUES: THE SAFETY IMPLICATIONS Off-target mutations : is the reward worth the risk? One problem is that large genomes may contain multiple DNA sequences identical or highly homologous to intended target DNA sequence. CRISPR/Cas9 may also cleave these unintended sequences causing mutations which may cause plant tumors, cell death, etc. Accidental release of experimental species to the environment The species modified through a gene drive may carry drastic consequences to the ecosystem equilibrium and it may alter the ecosystem permanently. We do not fully understand the consequences of this.
CITED WORKS: Yin, K., Han, T., Liu, G., Chen, T., Wang, Y., Yu, A. Y., & Liu, Y. (2015, October 09). A geminivirus-based guide RNA delivery system for CRISPR/Cas9 mediated plant genome editing. Retrieved July 30, 2017, from https://www.nature.com/articles/srep14926 Bomgardner, M. M. (n.d.). CRISPR: A new toolbox for better crops. Retrieved July 30, 2017, from http://cen.acs.org/articles/95/i24/CRISPR-new-toolbox-better-crops.html Jose, /. S. (2016, December 31). Genome editing: an introduction to CRISPR/Cas9. Retrieved July 30, 2017, from http://blog.globalplantcouncil.org/future-directions/genome-editing-crisprcas9/ Chavarriaga-Aguirre, P., Brand, A., Medina, A., Pr as, M., Escobar, R., Martinez, J., . . . Tohme, J. (2016, August 12). The potential of using biotechnology to improve cassava: a review. Retrieved July 30, 2017, from https://link.springer.com/article/10.1007/s11627-016-9776-3