Diastereoselective Synthesis Using Felkin-Anh Model
The diastereoselective synthesis process involves the strategic manipulation of stereochemistry using the Felkin-Anh model. By understanding the interactions between different substituents and the carbonyl group, chemists can control the outcome of reactions to favor specific diastereomers. This approach, as demonstrated in the provided images and descriptions, showcases the importance of spatial orientation in achieving desired stereochemical outcomes in organic synthesis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
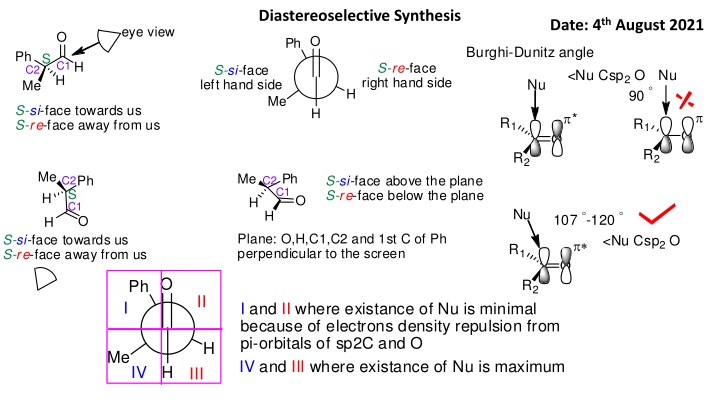
Diastereoselective Synthesis Date: 4th August 2021 eye view O O Ph Burghi-Dunitz angle Ph S C1 H S-re-face right hand side S-si-face left hand side C2 <Nu Csp2O Nu H Nu Me 90 H Me S-si-face towards us S-re-face away from us H * R1 R1 O O R2 R2 Me H Me Ph C1 Ph S-si-face above the plane S-re-face below the plane C2 C2 S C1 O H Nu H 107 -120 O H Plane: O,H,C1,C2 and 1st C of Ph perpendicular to the screen S-si-face towards us S-re-face away from us <Nu Csp2O * R1 O O Ph R2 II I I and II where existance of Nu is minimal because of electrons density repulsion from pi-orbitals of sp2C and O IV and III where existance of Nu is maximum H Me IV H III
Et Mg Br O Ph EtMgBr H polar solvent Me H Felkin-Anh Model Larger electron density substituent suppose to be perpendicular to the plane of the Carbonyl group O O H Me S-re-face right hand side S-si-face left hand side S-re-face right hand side S-si-face left hand side Ph Ph Me H H H Nguyen Trong Anh & Odile Eisenstein Tetrahedron Lett. 1976, 17, 155. Ch rest, M.; Felkin, H.; Prudent, N. Tetrahedron Lett.1978, 18, 2199
O H S-re-face right hand side S-si-face left hand side Ph Me Br H Br Mg O H Mg O H Ph S-re-face right hand side S-si-face left hand side Ph Me H Et encounter electron density repulsion from Me group IV Et encounter electron density repulsion from Ph group Et Me III H III Et IV favoured S-re-face attack S-si-face attack Br Mg Br eye view O O Br OH Mg H Ph H S O OH C1 H2O H Mg C2 H Ph H Br Ph H OH Me H2O Ph Et Et H Ph H Me Mg Me S-si-face towards us S-re-face away from us Me Et OH H Me C2- clock wise 30 Et C2- clock wise 30 H view axis is perpendicular to C1-C2 bond and alpha C is on left hand side carbonyl carbon is on right hand side
OH OH H H Ph Ph Et H Me Et C2- clock wise 30 H Me C2- clock wise 30 Ph OH Ph OH H Et H Me Et H Me H C1- anticlock wise 30 C1- clock wise 30 Ph Ph view this molecule from left hand side and our view axis is perpendicular to C1-C2 bond and alpha C suppose to be on left hand side and hydroxyl carbon suppose to be on right hand side OH R H OH H H HO Ph S H S HO S C1 Et C2 H Ph H Me Me C1 H Et C2 Me eye view Et Et H eye view major diastereomers Me
O Me S-re-face right hand side S-si-face left hand side Ph Br H Br H Mg O Mg O Me Me S-si-face left hand side S-re-face right hand side Et encounter electron density repulsion from Ph group S-re-face attack Br Ph Ph H H III H H Et IV III IV Et encounter electron density repulsion from H most favoured approach of Nucleophile to carbonyl carbon Et S-si-face attack Br Br OH Mg Mg Br Me Mg OH O O Me Me Me OH Ph Mg H2O H2O Et Ph Ph H Ph OH H H Et H H Et H C2- anticlock wise 30 Et H H C2- anticlock wise 30
OH OH Me Me Ph Ph Et H H Et H H C2- anticlock wise 30 C2- anticlock wise 30 Ph OH HO Ph Me Me H H Et Et H H C1- anticlock wise 30 C1- clock wise 30 Ph Ph H HO OH H Me H OH R Me H H eye view H S Et HO S eye view Et Ph S Ph C1 Et C2 C1 Et C2 H Me H Me major diastereomers