Effective SHTM Grants Workflow for Proposal Submission
"Learn a five-day deadline process for submitting proposals effectively, including notifying key individuals, developing proposal components, and ensuring timely approvals for successful submissions."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
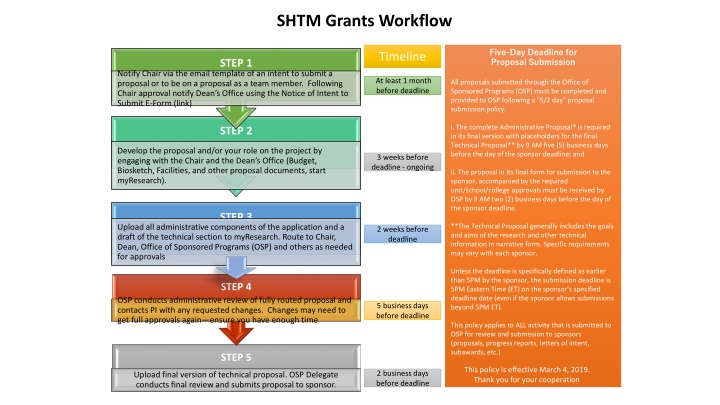
SHTM Grants Workflow Five-Day Deadline for Proposal Submission Timeline STEP 1 Notify Chair via the email template of an intent to submit a proposal or to be on a proposal as a team member. Following Chair approval notify Dean s Office using the Notice of Intent to Submit E-Form (link) At least 1 month before deadline All proposals submitted through the Office of Sponsored Programs (OSP) must be completed and provided to OSP following a "5/2 day" proposal submission policy: i. The complete Administrative Proposal* is required in its final version with placeholders for the final Technical Proposal** by 9 AM five (5) business days before the day of the sponsor deadline; and STEP 2 Develop the proposal and/or your role on the project by engaging with the Chair and the Dean s Office (Budget, Biosketch, Facilities, and other proposal documents, start myResearch). 3 weeks before deadline - ongoing ii. The proposal in its final form for submission to the sponsor, accompanied by the required unit/school/college approvals must be received by OSP by 9 AM two (2) business days before the day of the sponsor deadline. STEP 3 **The Technical Proposal generally includes the goals and aims of the research and other technical information in narrative form. Specific requirements may vary with each sponsor. Upload all administrative components of the application and a draft of the technical section to myResearch. Route to Chair, Dean, Office of Sponsored Programs (OSP) and others as needed for approvals 2 weeks before deadline Unless the deadline is specifically defined as earlier than 5PM by the sponsor, the submission deadline is 5PM Eastern Time (ET) on the sponsor s specified deadline date (even if the sponsor allows submissions beyond 5PM ET). STEP 4 OSP conducts administrative review of fully routed proposal and contacts PI with any requested changes. Changes may need to get full approvals again ensure you have enough time 5 business days before deadline This policy applies to ALL activity that is submitted to OSP for review and submission to sponsors (proposals, progress reports, letters of intent, subawards, etc.) STEP 5 This policy is effective March 4, 2019. Thank you for your cooperation 2 business days before deadline Upload final version of technical proposal. OSP Delegate conducts final review and submits proposal to sponsor.
Administrative Proposal Checklist Cover Page 1. Budget 2. Budget Justification Facilities and Resources 3. 4. Biosketches for Key Personnel 5. Current and Pending Support (if applicable) 6. Subrecipient Documentation 7. Other documents as required by sponsor 8. The Administrative Proposal generally includes the application cover page, budget, budget justification, institutional resources section, bio-sketches of all key personnel, current and pending support, subrecipient documentation, and any other business or administrative materials required by the sponsor. The administrative portion of the proposal encompasses all content excluding the technical portion. Specific requirements may vary with each sponsor.
Sub award Requirements (collaborations) Subrecipient Commitment Form 1. Letter of Intent to Establish Consortium Agreement 2. Budget 3. Budget Narrative (Justification) 4. Biosketch (CV) Formatted for Senior Key Personnel 5. Indirect Cost & Fringe Benefits Rate Agreements 6. Scope of Work Template 7.
myResearch Funding Proposal Checklist Funding Proposal Proposal Description & Contacts 1.0Short title of proposal. 2.0Principal Investigator Income Fund Reimbursable - Salary Offset (IFR)? if yes IFR account number is required. If you are creating this Funding Proposal on behalf of a PI make sure to add yourself as a Departmental Research Coordinator (Q.3) or Department Administrative Contact (Q. 4) BEFORE you save or continue from this page. 5.0Select Direct Sponsor: 6.0 Are there other personnel associated with this funding proposal? Note: Additional Personnel added to this proposal flood automatically to the budget. Personnel cannot be added manually to the budget. Please use TBD for unamed personnel General Proposal Information 1.0Type of Application and Type of Sponsor Selected. 2.0Modular budget ( only applies to NIH) 4.0Indicate how the forms will be submitted to the Sponsor: Please check Other as we are not yet using S2S 5.0Instrument Type: 6.0Describe the purpose of this project: 7.0Is this a Clinical Trial? 8.0Is this a multi-PI Submission? 9.0Is this an on campus submission, an off campus submission, or both? Research Department Determination 1.0Select the Submitting Department: Compliance Review 1.0 For each item listed below, indicate if it is involved in this project: Select all compliance items that are needed for your proposal 2.0Does the project involve (a) classified research (b) proprietary research (c) controlled unclassified information or (d) use or development of export controlled items or information? 3.0Does this project provide data or services to, conduct any transaction with, or require travel to an embargoed country as defined by the Office of Foreign Asset Controls, such as Cuba, Iran, North Korea, Sudan or Syria? Commitment of Additional Resources 2.0Does this research involve the use of Veterans Administration s patients, personnel and/or facilities? Program Classification 2.0This proposal is related to: Answer each question Continued
General Submission Information 2.0Required Routing Documents Is this a Clinical Trial? Y/N Upload documents as directed. Mandatory Non-Clinical Trial Docs Abstract/SOW, Facilities Statement, and Budget Justification or a fully copy of your proposal, as appropriate Mandatory Clinical Trial documents: Protocol, Informed Consent Form, and Facilities Statement and Final Approved Budget Submission Dates 1.0Application submission deadline (if there is no sponsor deadline, indicate the date you would like to submit): 3.0 Expected Start Date: Budget Periods: Automatically defaults to 5 years - Adjust to you proposal needs by Removing or Adding budget periods. Intellectual Property Questions Complets all questions Credit Distribution Complete Section with credit distributed as decided Budget General Budget Information 1.0Budget title: 2.0Principal Investigator for this budget: 3.0Does this budget use the standard indirect cost base and rates? 5.0Will this budget have cost sharing? Personnel Costs Complete with personnel. General Costs Complete with other direct costs categories.