Einsteinium: Synthetic Element Discovery & Uses
Found in 1952 from hydrogen bomb debris, einsteinium is a synthetic element classified as actinides metal series. Named after Albert Einstein, its most stable isotope has a half-life of 471.7 days. Despite production challenges, ongoing nuclear reactions allow for further study in scientific research.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Einsteinium Element 99
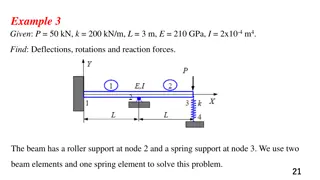
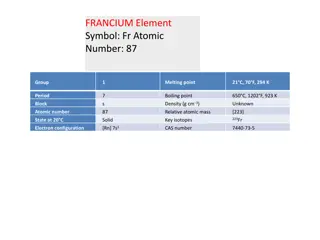
Einsteinium Element Symbol: Es Einsteinium is a synthetic element from the actinides metal series, it is the seventh transuranic element.
Why einsteinium? Einsteinium is called Einsteinium because it was found from the component of the debris from the first hydrogen bomb in 1952.
Discovery Einsteinium was found by Albert coincidence Ghiorso, in 1952 from the debris of the first hydrogen bomb.
Uses To this day there have been no found uses for Einsteinium
How its found The isotope they discovered, einsteinium-253, has a half-life of about 20 days and was produced by combining 15 neutrons with uranium-238, which then underwent seven beta decays. Today, einsteinium is produced though a lengthy chain of nuclear reactions that involves bombarding each isotope in the chain with neutrons and then allowing the resulting isotope to undergo beta decay. Einsteinium's most stable isotope, einsteinium-252, has a half-life of about 471.7 days. It decays into berkelium-248 through alpha decay or into californium-252 through electron capture. Since only small amounts of einsteinium have ever been produced, it currently has no uses outside of basic scientific research. (3)
Citations 1.https://en.wikipedia.org/wiki/Einsteinium 2.https://www.lenntech.com/periodic/elements/es.htm 3.https://education.jlab.org/itselemental/ele099.html