Electrodeposition and Electrospinning Techniques
The final size distribution of electrodeposits is influenced by the kinetics of nucleation and growth, while electrospinning is a versatile method for producing nanofibers with various applications in areas such as drug delivery, tissue engineering, and energy-related fields.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
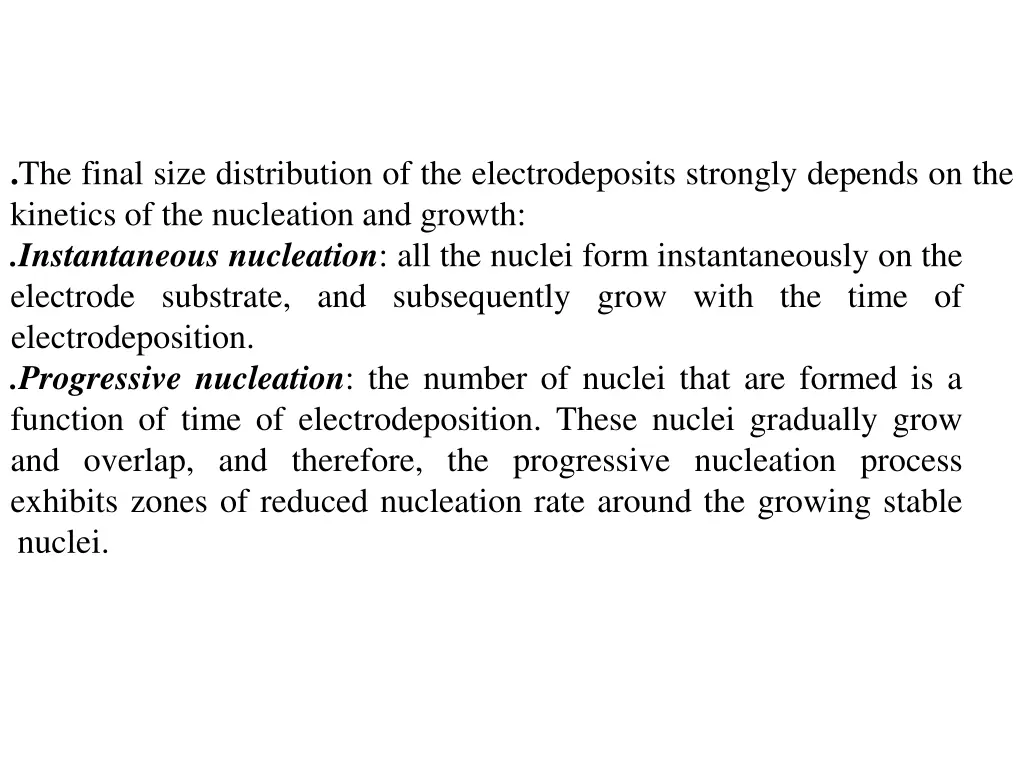
.The final size distribution of the electrodeposits strongly depends on the kinetics of the nucleation and growth: .Instantaneous nucleation: all the nuclei form instantaneously on the electrode substrate, and subsequently grow with the time of electrodeposition. .Progressive nucleation: the number of nuclei that are formed is a function of time of electrodeposition. These nuclei gradually grow and overlap, and therefore, the progressive nucleation process exhibits zones of reduced nucleation rate around the growing stable nuclei.
3. Electrospinning method A large number of advanced techniques have been employed to fabricate 1-D nanostructure, but electrospinning seems to be the simplest and most versatile method. Electrospinning is a broadly used technique for electrostatic fiber formation. In this method electrical force is utilized to produce polymer fibers with diameters ranging from few nanometers to several micrometers using polymer solutions of both natural and synthetic polymers. It is the process by which continuous nanofibers are produced with high surface area- to-volume ratio.
Basically, an electrospinning system consists of three major components: a high voltage power supply, a spinneret (e.g., a pipette tip) and a grounded collector (usually a metal screen, plate, or rotating mandrel) and utilizes a high voltage source to inject charge of a certain polarity into a polymer solution or melt, which is then accelerated towards a collector of opposite polarity. In this process, a polymer solution is injected from a needle in the presence of electric field. A DC voltage in the range of several tens of kVs is necessary to generate electric field during the electrospinning. When the applied electric field overcomes the surface tension of the liquid a continuous jet is ejected which upon subsequent solvent evaporation and bending produces nanofibers on the collector surface
This method has following advantages: (i) the ability to produce thin fibers with diameters in the micrometer and nanometer ranges; (ii) one-step forming of the two-or three dimensional nanofiber network assemblies and (iii) applicability for a broad spectrum of molecules, such as synthetic and biological polymers. Recently, various applications of electrospun fibers are being carried out, as these fibers have high surface to volume ratio, very high porosity and enhanced physico- mechanical properties.
Applications of electrospun nanofibers in drug delivery and tissue engineering. The use of electrospinning technique for biomedical applications. The use of electrospinning to create materials suited for four major energy-related applications i.e. fuel cells, dye-sensitized solar cell, Li-ion capacitors. batteries and super
Carbon-based materials In nature, there are some pure materials that have strikingly different properties even though they are made up of the same atoms. For instance, graphite and diamond (Figure 1): two very popular materials, one used conventionally in pencils and the other in jewellery. These two materials could not be more different: graphite is soft, light, flexible, and conducts electricity while diamond is extremely strong, hard and does not conduct electricity. Both materials are made of carbon atoms linked through strong bonds (covalent): in graphite, each carbon atom uses three out of its four electrons to form single bonds with its neighbours, forming a linear sheet, whereas, in diamond, each carbon atom uses all its four electrons to form four single bonds, resulting in a 3D structure. The different properties of graphite and diamond are a consequence of the different way the carbon atoms in the materials are bonded together.
Figure 1: The two allotropes of carbon and their respective chemical structure: (left, top and bottom) diamond; (right, top and bottom) graphite
Graphite and diamond are two pure forms (allotropes) of carbon. In 1985, a new allotrope of carbon was discovered formed of 60 atoms of carbons linked together through single covalent bonds arranged in a highly symmetrical, closed shell that resembles a football. This material was officially named Buckminsterfullerene and is often referred to as buckyball, fullerene or simply C60. Since its discovery, fullerenes with 70, 80 and even more carbon atoms have been discovered. In the early 1990s, an incredible new carbon form was discovered: carbon nanotubes. These appear to be like graphite sheets rolled up with fullerene-type end caps, but have totally different properties compared to graphite. Figure 2 shows different forms of carbon allotropes (images (d) and (h) are structures of C60 and a nanotube, respectively).
Figure 2: Eight allotropes of carbon: (a) diamond; (b) graphite; (c) lonsdaleite; (d) C60; (e) C540; (f) C70; (g) amorphous carbon; and (h) a carbon nanotube