Electrolytes and Conductivity in Solutions
Learn about electrolytes, including their classification and role in conducting electricity in solutions. Explore the differences between electrolytes and nonelectrolytes and how they affect current conductivity.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
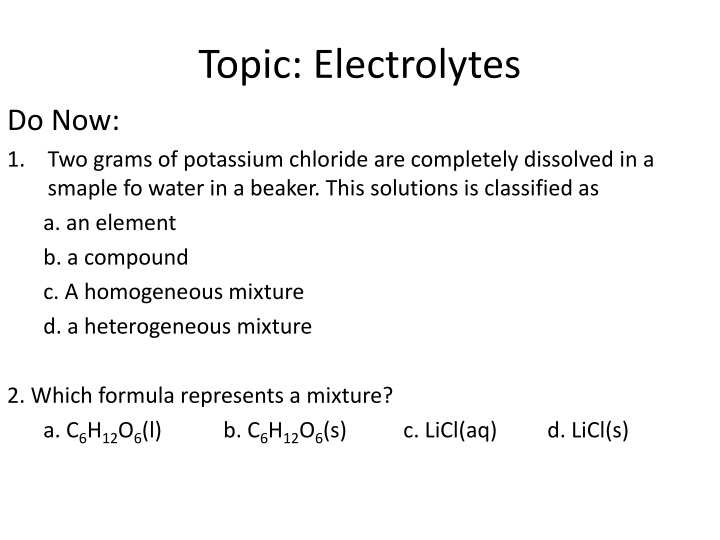
Topic: Electrolytes Do Now: 1. Two grams of potassium chloride are completely dissolved in a smaple fo water in a beaker. This solutions is classified as a. an element b. a compound c. A homogeneous mixture d. a heterogeneous mixture 2. Which formula represents a mixture? a. C6H12O6(l) b. C6H12O6(s) c. LiCl(aq) d. LiCl(s)
Electricity What do you need to conduct electricity? Mobile, charged particles!!!!
Electrolyte = ions in solution substance that dissolves in water to form solution that conducts electricity - Soluble salts (ionic compounds from table F) -Bases (metal ion + OH-) and (NH3 NH4+ + OH-) - Acids (start with H except H2O H2O2 or end in COOH) Nonelectrolyte substance that dissolves in water to form solution that does not conduct electricity - basically all covalent except acids and bases
Electrolyte vs. Nonelectrolyte Which solution conducts a current?