Electronic Structure and Chemical Bonding in Quantum Mechanics
Explore the principles of electronic structure and bonding through Schrödinger's equation, Born-Oppenheimer approximation, molecular solids, extended solids, and potential energy terms in solid-state systems.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
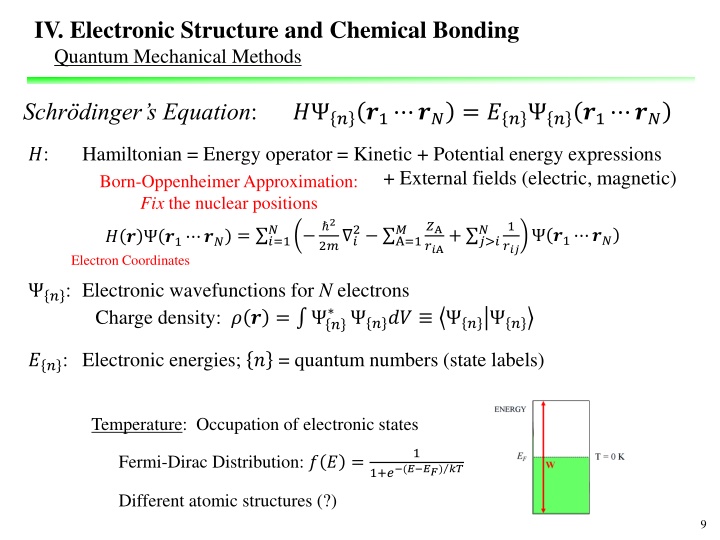
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods Schr dinger s Equation: ? ? ?1 ?? = ?? ? ?1 ?? ?: Hamiltonian = Energy operator = Kinetic + Potential energy expressions + External fields (electric, magnetic) Born-Oppenheimer Approximation: Fix the nuclear positions 2 2? ? ?A ??A+ ?>? 1 ? 2 A=1 ? ? ?1 ?? ? ? ?1 ?? = ?=1 ? ? ?1 ?? Electron Coordinates ??? ?: Electronic wavefunctions for N electrons Charge density: ? ? = ? ??? ? ? ??: Electronic energies; ? = quantum numbers (state labels) Temperature: Occupation of electronic states 1 Fermi-Dirac Distribution: ? ? = (? ??) ?? 1+? Different atomic structures (?) 9
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Structure Modelling Strategies Schr dinger s Equation: ? ? ?1 ?? = ?? ? ?1 ?? A solid is a molecule with a quasi-infinite number (ca. 1023) of atoms. A solid is a molecule with a quasi-infinite number (ca. 1023) of atoms. Molecular Solids: Use molecular entities (as in gas phase); packing effects? Extended Solids: How to make the problem tractable? Amorphous (glasses silicates, phosphates): use molecular fragments, tie off ends with simple atoms, e.g., H Aperiodic (incommensurately modulated, quasicrystals): use crystalline approximants Crystalline (metals, semiconductors, salts): use translational symmetry and space groups elegant simplification! Mixed Site Occupancies? use subgroups or superstructures or Coherent Potential Approximation 10
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Potential Energy Terms ? ? ? 2 2? ? ?A ??A 1 ??? Schr dinger s Equation: for electrons in a solid 2 + ?1 ?? = ?? ?1 ?? Multi-Electron ?=1 A=1 ?>? Kinetic Potential One-Electron Independent Electron (Hartree) Approximation: e (?) moves in the electrostatic field of the other e s Wavefunctions and energies determined from a single-particleSchr dinger s equation: ?1 ?? = ?1?1 ?2?2 ???? ? 2 ? ? = ??? ?=1 ? 2 eff Hartree Potential 2 2? ? ???? = ? ?? ???? ?A ??A 2 ??? + ???? ???? = ?? ???? ?? ?? A=1 Self-Consistency needed to solve these equations Valence ? ? on Atoms (Ions) Challenge: How best to model the potential energy? Simple metals (Na, Mg, Cu, Zn): Free-Electron Nearly-Free-Electron Pseudopotentials Transition metals, Semiconductors, Insulators: Tight-Binding / L.C.A.O. Pseudopotentials Isotropic Potentials Localized Potentials Valence ? ? in Bonds 11
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Potential Energy Terms ? ? ? 2 2? ? ?A ??A 1 ??? Schr dinger s Equation: for electrons in a solid 2 + ?1 ?? = ?? ?1 ?? ?=1 A=1 ?>? Kinetic Potential One-Electron Potential: A-e attraction + Hartree potential = ?eff One-Electron Potential ???+ ???? Two-Electron Potential: Exchange (?) and Correlation (?) = ???? ?? Two-Electron Potential: Exchange (?) and Correlation (?) Two-Electron Potential Spin Spin Charge Charge ? ? = ? ? + ? ? ? ? (Metals) Free Electrons: Hartree-Fock (Slater Determinant) Free Electrons ? 1 ?0 ?1/3 ?? ~ ??? ??? as ?? ?? DOS 0 states at ?? Nonmetallic! 1/2 ???? ~ ??2 ?? ?? ?? Spin Only ? ?? ?? = ? ?? = ? Exclusion Hole 1/3 = ? ? ? Average over all occupied states ? ?0 ?? ?? Fermi (Coulomb and Exchange) Hole +1 background charge not screened by ? ? Quasi-particle: ? and +1 background Forerunner of the Local Density Approximation (LDA) 12
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Potential Energy Terms ? ? ? 2 2? ? ?A ??A 1 ??? Schr dinger s Equation: for electrons in a solid 2 + ?1 ?? = ?? ?1 ?? ?=1 A=1 ?>? Kinetic Potential One-Electron Potential: A-e attraction + Hartree potential = ?eff One-Electron Potential ???+ ???? Two-Electron Potential: Exchange (?) and Correlation (?) = ???? ?? 1/3 LDA (Local Density Approx.): ???? ~ ? ? ? ???= ? ? ???? ? ?? GGA (Generalized Gradient Approx.): ???? = ? ? ? ,?? ? Hubbard Model ?0+ ? ? Magnetic Nonmetal Metal ?0 ? ~ ?? ?? = On-site e e Repulsion Mott-Hubbard Transition ?/? Increases ? = 0 13
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Computational Approaches Electronic Structure of Si (Diamond Structure): 10 8 Energy 6 4 2 Fermi Level 0 (eV) -2 -4 -6 -8 -10 -12 -14 L X W L K ??? Electronic Band Structure Electronic Density of States How are these diagrams obtained? What can we learn from the information? 14
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Computational Approaches Schr dinger s Equation: ??? = ?????? ? Basis Functions Atomic Orbitals Plane Waves ???= ???? ????? ??? ?? ??= ?? ??? ?? ??? ?? ?? Intra-Site Integrals ? ? ? Matrix 1 Rydberg = 13.6 eV 1 Rydberg = 1313 kJ/mol 1 eV = 96.5 kJ/mol 1 eV = 23.1 kcal/mol ??? ?? ??? ?? Inter-Site Integrals ?? Empirical / Semi-empirical: Integrals assigned values based on experimental data (AO energies) or simple algorithms (resonance integrals AO overlap) H ckel, Extended H ckel (EHT); Neglect of Differential Overlap (NDO methods) Minimal basis sets (only valence AOs) computationally fast Ignore explicitly two-electron potential energy terms Results over-estimate HOMO-LUMO gaps for insulators / semiconductors Poorly optimizes interatomic distances 15
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Computational Approaches Schr dinger s Equation: ??? = ?????? ? Basis Functions Atomic Orbitals Plane Waves ???= ???? ????? ??? ?? ??= ?? ??? ?? ??? ?? ?? Intra-Site Integrals ? ? ? Matrix 1 Rydberg = 13.6 eV 1 Rydberg = 1313 kJ/mol 1 eV = 96.5 kJ/mol 1 eV = 23.1 kcal/mol ??? ?? ??? ?? Inter-Site Integrals ?? First Principles: Calculate all integrals using physical models Hartree-Fock (HF): incorporates anti-symmetrized wavefunctions as Slater determinants Useful for insulators & semiconductors; weaker for metals (exchange; single determinant; ) Does not fully account for correlation assumes electrons move in a mean field potential calculated energies tend to be too high Need multiple Slater determinants (configuration interaction) to account for correlation 16
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Computational Approaches Schr dinger s Equation: ??? = ?????? ? Basis Functions Atomic Orbitals Plane Waves ???= ???? ????? ??? ?? ??= ?? ??? ?? ??? ?? ?? Intra-Site Integrals ? ? ? Matrix 1 Rydberg = 13.6 eV 1 Rydberg = 1313 kJ/mol 1 eV = 96.5 kJ/mol 1 eV = 23.1 kcal/mol ??? ?? ??? ?? Inter-Site Integrals ?? First Principles: Calculate all integrals using physical models Density Functional Theory (DFT): reformulates the problem using electron density ? ? Well-suited for ground-state (collective)properties, e.g., structure optimization, charge density Band gaps underestimated for semiconductors Exchange-Correlation explicitly included (approximations): Local Density Approx. (LDA): ???? expressed by form using local electron density Local Spin Density Approx. (LSDA): like LDA but different potentials and basis functions for spin- up / spin-down wavefunctions Hubbard Correlation (LDA + U): includes on-site ? parameter for localized electrons, e.g., in compounds with 4? and certain 3? metals 17
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Computational Approaches Schr dinger s Equation: ??? = ?????? ? Basis Functions Atomic Orbitals Plane Waves ???= ???? ????? ??? ?? ??= ?? ??? ?? ??? ?? ?? Intra-Site Integrals ? ? ? Matrix 1 Rydberg = 13.6 eV 1 Rydberg = 1313 kJ/mol 1 eV = 96.5 kJ/mol 1 eV = 23.1 kcal/mol ??? ?? ??? ?? Inter-Site Integrals ?? VASP, WIEN2k, CRYSTAL, QUANTUM ESPRESSO First Principles CODES: Free Electron: No Potential Nearly Free Electron (NFE) Apply periodic potential of nuclear sites Pseudopotentials (PSP) Apply core-valence orthogonalization Tight Binding ( MO ): Atomic Potentials Linear Combination of Basis Functions Adapted to symmetry ( SALCs ) Bloch functions vs. Wannier functions Spatially delocalized Spatially localized Cellular Approaches Divide structure into cells Atomic-like vs. Constant potentials 18
IV. Electronic Structure and Chemical Bonding Quantum Mechanical Methods: Computational Approaches Cellular Approach Divide the structure into Wigner-Seitz (WS) cells ??? must be continuous at boundaries Atomic-like Orbitals ??? = ?,???????,? ????,? ; ??? = ?,???????,? ????,? ; ? ??? ? ??? ? ??? ? ??? M M ????; ????; Plane Waves M M M ??? = radius of sphere M M M M M Free Electron-like (Constant) Potential M M M M M M M Atomic-like (Spherical) Potential Polyhedral shapes of WS cells make this solution challenging, so Divide potential energy space into 2 regions Simplifications (Approximation) Atomic-Sphere-Approximation (ASA) Overlapping spheres Linearized Radial Functions (inc. speed) ??? ?? ?? Linear Muffin-Tin Orbital (LMTO) 2 ??? = ???? + ? ?? + ? ? ?? 19