Empirical and Molecular Formulas in Chemistry
Explore the concepts of empirical and molecular formulas in chemistry, including their differences, determination methods, and molecular formula calculations. Learn how to calculate molecular formulas from percentage composition data and molecular mass to understand chemical compounds better.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
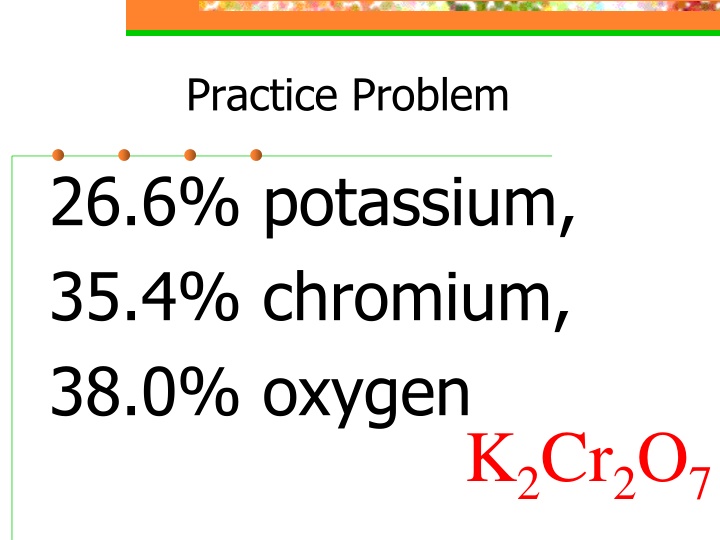
Practice Problem 26.6% potassium, 35.4% chromium, 38.0% oxygen K2Cr2O7
Lesson 27 Molecular Formula Objectives: - The student will calculate molecular formulas from an empirical formula and a molecular mass. - The student will calculate empirical and molecular formulas from percentage composition data and a molecular mass.
I. Empirical Formulas A. Up to this point, we have represented formulas in two different ways: i. Lewis Structures ii. Chemical formulas B. Now, we will look at the difference between empirical formulas and molecular formulas
II. Empirical formulas are determined experimentally A. Many times when scientists discover a new compound, they determine the empirical formula from composition data. B. They can also use other methods to determine the molecular mass of the compound present. This can be used to determine a molecular formula.
III. Molecular formulas A. An empirical formula just tells you how much of each element is present, but in lowest number ratios. BMolecular formula gives type and actual number of elements in a compound. C. Many different compounds can have the same empirical formula, but they may not have the same molecular formula. D. Also, many times the empirical formula for a compound is the same as the molecular formula sometimes, it is not.
E. Examples all these have CH2O as their empirical formula 1. Formaldehyde CH2O poisonous 2. Acetic Acid (vinegar) C2H4O2 edible 3. Glucose C6H12O6 sugar
IV. Molecular formulas are determined from molar mass A. Molecular formulas are multiples of the empirical formulas B. X (empirical formula) = molecular formula C. X will be a whole number. D. To determine X, divide the molecular formula mass by the empirical formula mass
Molecular Formula Calculations: In calculations of molecular formula, you will be given percent compositions and the molecular weight of the entire compound. 1. 2. 3. 4. Calculate the empirical formula as shown earlier. Calculate the empirical mass of the empirical formula. Divide the given molecular mass by the empirical mass. The answer should be a whole number, multiply this through the subscripts of the empirical formula to get the molecular formula.
Example: Problem 1 level 1 on page 206 26.7% C = 26.7g C (1 mole/ 12.011)= 2.22296 moles C 2.2% H= 2.2g H(1 mole/ 1.0079) = 2.182756 moles H 71.1%O=71.1g O(1 mole/15.999)= 4.444 moles O
2.182756 is the smallest so divide all by this C 2.22296/2.182756 = 1.018 = about 1 H 2.182756/2.182756 = 1 O 4.444/ 2.182756 = 2.0359 = about 2 Use these numbers as the subscripts, giving you CHO2
Now take this formula to calculate the empirical mass C- 1 x 12.011 = H- 1 x 1.0079 = O- 2 x 15.999 = 12.011 1.0079 31.998 45.169 empirical mass
Now divide the molecular mass you were given in the problem, by the empirical mass that you calculated: 90/45.169 = 2 take this number and multiply all the subscripts in the empirical formula by it to get the molecular formula CHO2 becomes C2H2O4
Examples: A refrigerant, Freon-12, is composed of 9.93% Carbon, 58.64% Chlorine, and 31.43% Fluorine. What is its molecular formula is it has a molar mass of 120.91g/n? 1. % grams 2. Grams moles Divide by smallest number of moles 4. Determine empirical formula 5. Find empirical mass 6. Divide molecular mass by empirical mass 7. Multiply subscripts of empirical formula by the answer in step 6 3.
FIND THE EMPIRICAL FORMULA AND MOLECULAR FORMULA A compound has a make up of: 23.88 % Sodium 42.89% Phosphorus 33.23% Oxygen And has a Molecular Mass of 1,440 g/mol
Level 1 A compound has the following percentage composition: 26.7% carbon, 2.2% hydrogen, 71.1% oxygen. The molecular weight of this compound is 90. What is the compound s true formula? A certain compound was analyzed and found to have the following composition: 54.6% carbon, 9.0% hydrogen, 36.4% oxygen. The true molecular weight for the compound is 176. What is the molecular formula of the compound? The percentage composition of ethane gas is 80.0% carbon and 20.0% hydrogen. The molecular weight for ethane is 30. What is the correct formula for this compound? Analysis of a compound shows that it consists of 24.3% carbon, 4.1% hydrogen, and 71.6% chlorine. The molecular weight of the compound is determined to be 89.8. What molecular formula corresponds to these data? An unknown compound is analyzed and found to consist of 49.0% carbon, 2.7% hydrogen, and 48.2% chlorine. Boiling point data suggest that the molecular weight of this compound is around 150. What molecular formula would you predict for this compound? A gaseous compound is found to have the following composition data: 30.5% nitrogen and 69.5% oxygen. The molecular weight of the gas is found to be 91.8. What molecular formula corresponds to these data? 1. 2. 3. 4. 5. 6.