Enthalpy Change Experiment Analysis in AP Chemistry Exam
Explore the 2018 AP Chemistry exam question focusing on determining the limiting reactant in an oxidation-reduction reaction through the analysis of concentrations and volumes of reactants used. Understand the process and reasoning behind identifying the limiting reactant in chemical reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
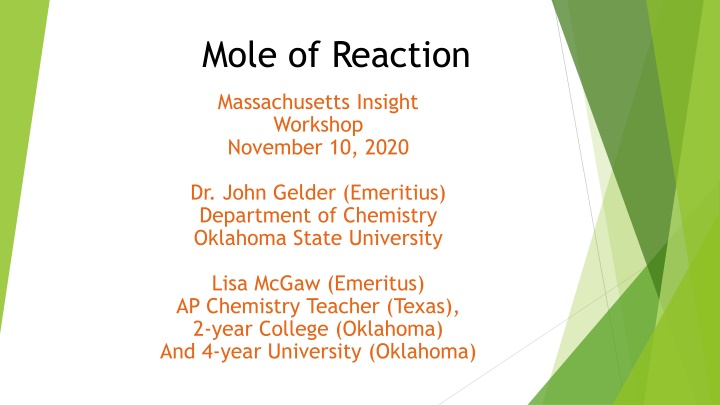
Mole of Reaction Massachusetts Insight Workshop November 10, 2020 Dr. John Gelder (Emeritius) Department of Chemistry Oklahoma State University Lisa McGaw (Emeritus) AP Chemistry Teacher (Texas), 2-year College (Oklahoma) And 4-year University (Oklahoma)
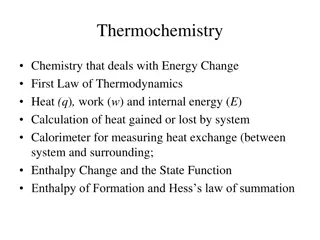
AP Chemistry Exam Equations for Thermochemistry
2018 AP Chemistry Exam Question 1 Slide 1 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) 1. A student performs an experiment to determine the value of the enthalpy change, H rxn, for the oxidation-reduction reaction represented by the balanced equation above. In the experiment, the student uses the solutions shown in the table below. Solution Na2S2O3(aq) NaOCl(aq) NaOH(aq) Concentration (M) 0.500 0.500 0.500 Volume (mL) 5 5 5 (c) Using the balanced equation for the oxidation-reduction reaction and the information in the table above, determine which reactant is the limiting reactant. Justify your answer.
2018 AP Chemistry Exam Question 1 Slide 2 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) 1. A student performs an experiment to determine the value of the enthalpy change, H rxn, for the oxidation-reduction reaction represented by the balanced equation above. In the experiment, the student uses the solutions shown in the table below. Solution Concentration (M) Na2S2O3(aq) 0.500 NaOCl(aq) 0.500 NaOH(aq) 0.500 Volume (mL) 5 5 5 (c) Using the balanced equation for the oxidation-reduction reaction and the information in the table above, determine which reactant is the limiting reactant. Justify your answer.
2018 AP Chemistry Exam Question 1 Slide 3 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) 1. A student performs an experiment to determine the value of the enthalpy change, H rxn, for the oxidation-reduction reaction represented by the balanced equation above. In the experiment, the student uses the solutions shown in the table below. Solution Concentration (M) Na2S2O3(aq) 0.500 NaOCl(aq) 0.500 NaOH(aq) 0.500 Volume (mL) 5 5 5 (c) Using the balanced equation for the oxidation-reduction reaction and the information in the table above, determine which reactant is the limiting reactant. Justify your answer.
2018 AP Chemistry Exam Question 1 Slide 4 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) 1. A student performs an experiment to determine the value of the enthalpy change, H rxn, for the oxidation-reduction reaction represented by the balanced equation above. In the experiment, the student uses the solutions shown in the table below. Solution Concentration (M) Na2S2O3(aq) 0.500 NaOCl(aq) 0.500 NaOH(aq) 0.500 Volume (mL) 5 5 5 (c) Using the balanced equation for the oxidation-reduction reaction and the information in the table above, determine which reactant is the limiting reactant. Justify your answer.
2018 AP Chemistry Exam Question 1 Slide 5 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) The solutions, all originally at 20.0 C, are combined in an insulated calorimeter. The temperature of the reaction mixture is monitored, as shown in the graph below. (d) According to the graph, what is the temperature change of the reaction mixture?
2018 AP Chemistry Exam Question 1 Slide 6 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) The solutions, all originally at 20.0 C, are combined in an insulated calorimeter. The temperature of the reaction mixture is monitored, as shown in the graph below. (d) According to the graph, what is the temperature change of the reaction mixture?
2018 AP Chemistry Exam Question 1 Slide 7 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) The solutions, all originally at 20.0 C, are combined in an insulated calorimeter. The temperature of the reaction mixture is monitored, as shown in the graph below. (d) According to the graph, what is the temperature change of the reaction mixture? (e) The mass of the reaction mixture inside the calorimeter is 15.21 g. (i) Calculate the magnitude of the heat energy, in joules, that is released during the reaction. Assume that the specific heat of the reaction mixture is 3.94 J/(g C) and that the heat absorbed by the calorimeter is negligible. qrxn = qsolution = (mass c T)solution qrxn =
2018 AP Chemistry Exam Question 1 Slide 8 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) The solutions, all originally at 20.0 C, are combined in an insulated calorimeter. The temperature of the reaction mixture is monitored, as shown in the graph below. (d) According to the graph, what is the temperature change of the reaction mixture? (e) The mass of the reaction mixture inside the calorimeter is 15.21 g. (i) Calculate the magnitude of the heat energy, in joules, that is released during the reaction. Assume that the specific heat of the reaction mixture is 3.94 J/(g C) and that the heat absorbed by the calorimeter is negligible. qrxn = qsolution = (mass c T)solution qrxn = (ii) Using the balanced equation for the oxidation-reduction reaction and your answer to part (c), calculate the value of the enthalpy change of the reaction, H rxn, in kJ/molrxn. Include the appropriate algebraic sign with your answer. Hrxn = qrxn /molrxn = Hrxn =
2018 AP Chemistry Exam Question 1 Slide 9 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) The solutions, all originally at 20.0 C, are combined in an insulated calorimeter. The temperature of the reaction mixture is monitored, as shown in the graph below. (g) Write the balanced net ionic equation for the given reaction.
2018 AP Chemistry Exam Question 1 Slide 10 Na2S2O3(aq) + 4 NaOCl(aq) + 2 NaOH(aq) 2 Na2SO4(aq) + 4 NaCl(aq) + H2O(l) The solutions, all originally at 20.0 C, are combined in an insulated calorimeter. The temperature of the reaction mixture is monitored, as shown in the graph below. (g) Write the balanced net ionic equation for the given reaction.
2017 AP Chemistry Exam Question 2 Slide 1 (b) Using the Lewis electron-dot diagrams of fulminic acid and isocyanic acid shown in the boxes above and the table of average bond enthalpies below, determine the value of H for the reaction of HCNO(g) to form HNCO(g).
AP Chemistry Exam Equations for Thermochemistry
Mathematical Equations NOT on AP Chemistry Exam Equations Pages H rxn = (Bond Energy(reactants)) (Bond Energy(products))
2017 AP Chemistry Exam Question 2 Slide 1 (b) Using the Lewis electron-dot diagrams of fulminic acid and isocyanic acid shown in the boxes above and the table of average bond enthalpies below, determine the value of H for the reaction of HCNO(g) to form HNCO(g). H rxn = (Bond Energy(reactants)) (Bond Energy(products))
2017 AP Chemistry Exam Question 2 Slide 2 (b) Using the Lewis electron-dot diagrams of fulminic acid and isocyanic acid shown in the boxes above and the table of average bond enthalpies below, determine the value of H for the reaction of HCNO(g) to form HNCO(g). H rxn = (Bond Energy(reactants)) (Bond Energy(products)) H rxn = H rxn = H rxn =