Enzyme Regulation: Control by Allostery and Phosphorylation in Glycogen Phosphorylase
This content delves into the regulatory mechanisms of glycogen phosphorylase through allostery and phosphorylation, highlighting the shift between active and inactive conformations. It also discusses the structural basis for cooperative substrate binding, the significance of multisubunit enzymes, and the control of activity via phosphorylation and dephosphorylation. Advantages of phosphorylation/dephosphorylation cascade systems over simple allosteric regulation are also explored.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
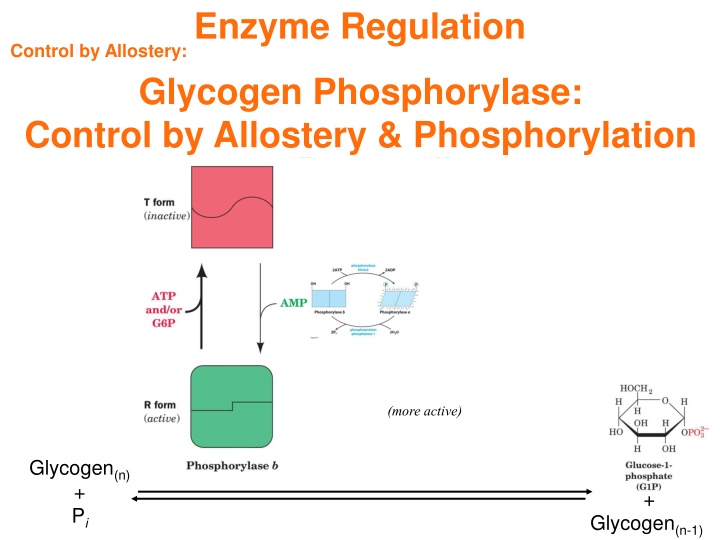
Enzyme Regulation Control by Allostery: Glycogen Phosphorylase: Control by Allostery & Phosphorylation (more active) Glycogen(n) + Pi + Glycogen(n-1)
Enzyme Regulation Control by Allostery: Glycogen Phosphorylase: Control by Allostery & Phosphorylation (more active) Glycogen(n) + Pi + Glycogen(n-1)
Enzyme Regulation Control by Allostery: Rabbit Muscle Glycogen Phosphorylase (dimer) Covalent phosphoryl group (activating) Allosteric positive effector 45 Notice that these are both near the subunit-subunit interface and both add a phosphoryl group This is the R-state. Is there are T-state? Active site
Enzyme Regulation Control by Allostery: Rabbit Muscle Glycogen Phosphorylase Glycogen phosphorylase PDBids 8GPB and 7GPB Phospho-Ser AMP Active site
Enzyme Regulation Control by Allostery: Rabbit Muscle Glycogen Phosphorylase Glycogen phosphorylase PDBids 8GPB and 7GPB Phospho-Ser AMP Active site
Enzyme Regulation Key Concepts for Control of Enzyme Activity Allosteric effectors bind to multisubunit enzymes, such as aspartate transcarbamoylase, thereby inducing cooperative conformational changes that alter the enzyme s catalytic activity. Phosphorylation and dephosphorylation of an enzyme such as glycogen phosphorylase can control its activity by shifting the equilibrium between more active and less active conformations. You should be able to: Compare and contrast the actions of an allosteric effector, a competitive enzyme inhibitor, and a noncompetitive inhibitor. Explain the structural basis for cooperative substrate binding and allosteric control in ATCase. Why are such allosteric enzymes composed of more than one catalytic subunit? Describe how phosphorylation and dephosphorylation control the activity of glycogen phosphorylase. List some advantages of phosphorylation/ dephosphorylation cascade systems over simple allosteric regulation.