Exothermic and Endothermic Reactions
Explore the concepts of exothermic and endothermic reactions, differentiate between them, and learn how they affect temperature changes in the surroundings. Discover examples and key information to enhance your understanding of chemical reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
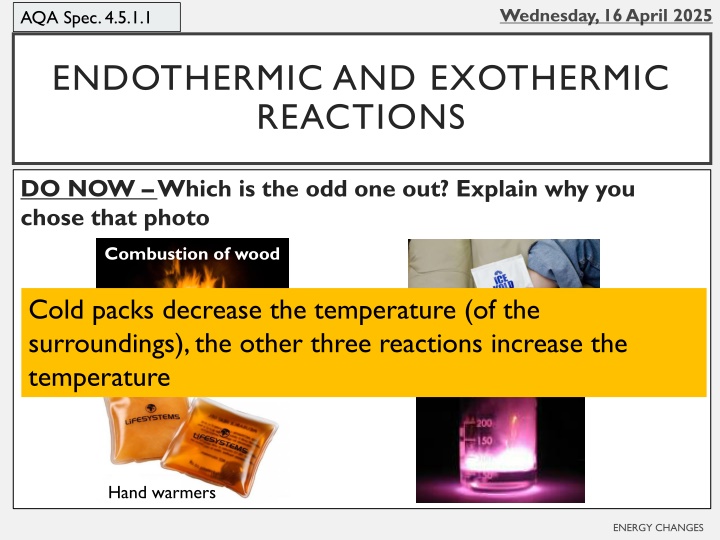
AQA Spec. 4.5.1.1 ENDOTHERMIC AND EXOTHERMIC REACTIONS DO NOW Which is the odd one out? Explain why you chose that photo Combustion of wood Cold packs decrease the temperature (of the surroundings), the other three reactions increase the temperature Cold packs Potassium and water Hand warmers
GOOD PROGRESS: DESCRIBE EXAMPLES OF ENDOTHERMIC AND EXOTHERMIC REACTIONS OUTSTANDING PROGRESS: EXPLAIN HOW TO USE AN EXPERIMENT TO CLASSIFY A REACTION
ACTIVITY 1 DEFINING ENDOTHERMIC AND EXOTHERMIC REACTIONS https://www.youtube.com/watch?v=eJXL0IrbtqE
Key ENDOTHERMIC AND EXOTHERMIC information An EXOTHERMIC reaction gives off energy to the surroundings Exothermic reactions INCREASE the temperature of the surroundings Examples include combustion, neutralisation and oxidation reactions The energy of the reactants is higher than the energy of the products An ENDOTHERMIC reaction takes in energy from the surroundings Endothermic reactions DECREASE the temperature of the surroundings Examples include thermal decomposition and the reaction between sodium carbonate and ethanoic acid The energy of the products is higher than the energy of the reactants
Use the coloured cards in your planners: Endothermic = red Exothermic = green MINI-PLENARY: WHAT TYPE OF REACTIONS ARE THE FOLLOWING? Self-heating cans EXOTHERMIC
Use the coloured cards in your planners: Endothermic = red Exothermic = green MINI-PLENARY: WHAT TYPE OF REACTIONS ARE THE FOLLOWING? Hand warmers EXOTHERMIC
Use the coloured cards in your planners: Endothermic = red Exothermic = green MINI-PLENARY: WHAT TYPE OF REACTIONS ARE THE FOLLOWING? Sports injury packs ENDOTHERMIC
