Exothermic vs. Endothermic Substances
Examine the differences between exothermic and endothermic substances in chemical reactions. Learn about examples of each type and their properties, as well as their daily uses. Explore how exothermic substances release energy as heat, while endothermic substances absorb thermal energy. Discover common substances like hydrochloric acid, sodium hydroxide, ammonium nitrate, and potassium chloride and their characteristics. Find out how these substances are utilized in various applications from power generation to refrigeration.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
EXOTHERMIC OR ENDOTHERMIC? BY MAJD KHALID AND MOHAMMED
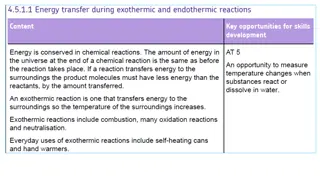
THE DIFFERENCE EXOTHERMIC Exothermic substances that release energy in the form of heat temperature of the surroundings to increase thus generating a positive change in temperature ENDOTHERMIC Endothermic substances that absorb or take in thermal energy temperature of the surroundings to decrease thus generating a negative change in temperature substances are substances are causing the causing the
LIST OF SUBSTANCES As previously stated there exists two types of substances in chemical reactions exothermic and endothermic so here are examples of the two. For exothermic we have hydrochloric acid and sodium hydroxide. Meanwhile for endothermic we have ammonium nitrate and potassium chloride
THEIR PROPERTIES AMMONIUM NITRATE POTASSIUM CHLORIDE HYDROCHLORIC ACID SODIUM HYDROXIDE Endothermic odorless - high melting point Endothermic strong oxidizing agent highly soluble Exothermic crystalline ate room temperature high melting point Exothermic very low pH highly reactive
THEIR DAILY USES EXOTHERMIC Exothermic products are generally used for generating providing energy applications, such systems, power generation, chemical reactions, and industrial processes ENDOTHERMIC Endothermic products are commonly used for cooling purposes, such as refrigeration, air conditioning, and heat absorption processes, as they absorb heat from their surroundings. heat various heating or in as in chemical