Exploring Atoms, Elements, and Molecules in Chemistry
Discover the fascinating world of atoms and elements, essential building blocks of matter. Uncover the composition of atoms, the arrangement of elements in the periodic table, and the formation of molecules and compounds. Dive into the atomic structure, atomic number, mass number, and the significance of important elements in biology. Enhance your understanding of chemical formulas and the essential role they play in chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
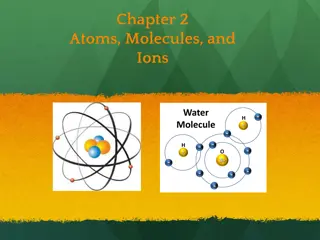
Vocabulary Atom Molecule Electron Nucleus Element Matter Proton Neutron Atomic Number Mass Number Atomic Symbol Periodic Table Neutral Chemical Formula Structural Formula Coefficient Subscript 2
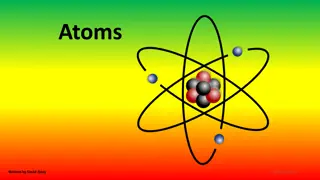
Atoms The basic units of matter Incredibly small Placed side by side, 100 million atoms would make a row only about 1 centimeter long about the width of your little finger! 3
What are Atoms made of? Made of particles called protons, neutrons, and electrons. Protons: positive (+) charge Electrons: negative (-) charge Neutrons: neutral (no charge) Normally have equal numbers of electrons and protons, making them neutral 4
Elements Pure substances made of only one type of atom Listed in the periodic table of elements Arranged in order of their atomic numbers An atom is the smallest unit of an element that has the properties of that element 5
Atomic Number & Mass Number Atomic number: the number of protons Typically the same as the number of electrons Mass number: the number of protons plus the number of neutrons 6
Practice Element Symbol Atomic # Mass # # of # of # of Protons Neutrons Electrons Helium He 2 4 2 Magnesium Mg 12 12 Zinc Zn 30 65 35 Bromine Br 80 45 35 Aluminum Al 13 14 8
Important Elements for Biology Five most abundant elements in living things are: 1. Hydrogen 10% 2.Oxygen 65% 3.Nitrogen 4% 4.Carbon 19% 5.Phosphorus 1% O C H H 9 N P
Molecules, Compounds, & Chemical Formulas Molecule: made when 2 or more of any atom are joined together Ex: O2, H2O Compound: a substance formed by the combination of two or more different elements Ex: H2O, C6H12O6 All compounds are molecules, but NOT all molecules are compounds Chemical Formula- written shorthand showing the composition of a compound 10
Chemical Formulas Coefficient: tells how many molecules of that substance Large number BEFORE the formula Subscript: goes with the element symbol preceding the number; tells how many atoms of that element within one molecule of the substance Small number WITHIN the formula 11
Examples Carbon 1 Hydrogen - 4 Hydrogen 12 Oxygen - 6 6 Carbon - 1 Oxygen - 2 Nitrogen - 1 Hydrogen - 3 12
Practice Example 2: 6CO2 What is the coefficient? _______ What is carbon s subscript? ________ What is oxygen s subscript? ________ How many molecules of this compound are represented by this formula? _________ How many atoms TOTAL are present in this molecule? ______ 18 6 1 2 6 13
Structural formulas Drawing shows: types of elements in the molecule number of atoms of each element arrangement of atoms and location of covalent bonds. Shows the two dimensional shape of the molecule water glucose 14