Exploring PHP - A Powerful Server-Side Scripting Language
PHP, Hypertext Preprocessor, is a widely-used open-source scripting language executed on the server. It allows for dynamic page content generation, file manipulation, form data handling, cookie management, database interaction, user access control, and data encryption. Learn about PHP syntax, file structure, and variable naming conventions to leverage its capabilities efficiently.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
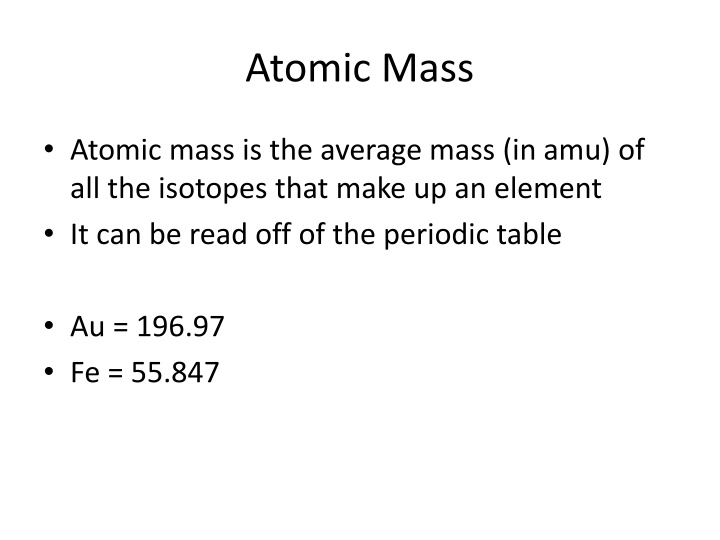
Atomic Mass Atomic mass is the average mass (in amu) of all the isotopes that make up an element It can be read off of the periodic table Au = 196.97 Fe = 55.847
Molecular Mass Molecular mass is the sum of the atomic masses of all the atoms in a compound. H2SO4 H 2 X 1.0079 = 2.0158 S 1 X 32.066 = 32.066 O 4 X 15.999 = 63.996 SUM = 98.0778
Mole Words can stand for numbers Pair Dozen Score Gross 144 2 12 20 MOLE 6.02 X 1023
Molar Mass Molar mass is the mass of one mole ( think one dozen) of the substance. For atoms the molar mass is the atomic mass expressed in grams. For molecules the molar mass is the molecular mass expressed in grams
Conversion Factors A fraction used to convert one unit to another The fraction has to be equivalent to one How many inches are in 1.13miles? 12inches 1foot 5280feet (1.13 miles)( )( ) = 71596.8 inch 1mile
Conversion Factors If you have 46g of Al, how many moles of Al do you have? 1mole 26.982g (46g)( ) = 1.7048moles = 1.7moles If you have 2.84moles of Al, how many grams of Al do you have? 26.982g 1mole (2.84moles)( ) = 76.6289g = 76.6g
2N2O54NO2 + O2 When N2O5 is heated, it decomposes: a. How many moles of NO2 can be produced from 4.3 moles of N2O5? 2N2O5 4NO2 + O2 = 4 moles 2 moles 4.3 moles X b. How many moles of O2 can be produced from 4.3 moles of N2O5? 2N2O5 4NO2 + O2 = 2 moles 1 mole 4.3 moles X 7
2N2O54NO2 + O2 When N2O5 is heated, it decomposes: a. How many grams of N2O5 were used if 210g of NO2 were produced? 2N2O5 4NO2 + O2 Find the molar mass of 2 moles of N2O5 and 4 moles of NO2. Then set up the given information as before 216.018g= 184.02g X 210g b. 75.0 grams of N2O5 can produce how many grams of O2? 2N2O5(g) 4NO2(g) + O2(g) 216.018g 31.98g = 75.0 g X 8