Exploring Symmetry and Crystallography in Nature, Arts, and Industry
"Delve into the world of symmetry in nature, arts, and industry through crystallography, exploring concepts like symmetry elements, rotoinversion, glide planes, screw axes, and axial combinations. Discover the beauty of symmetry in two and three dimensions, along with the fascinating geometric principles governing crystalline structures."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
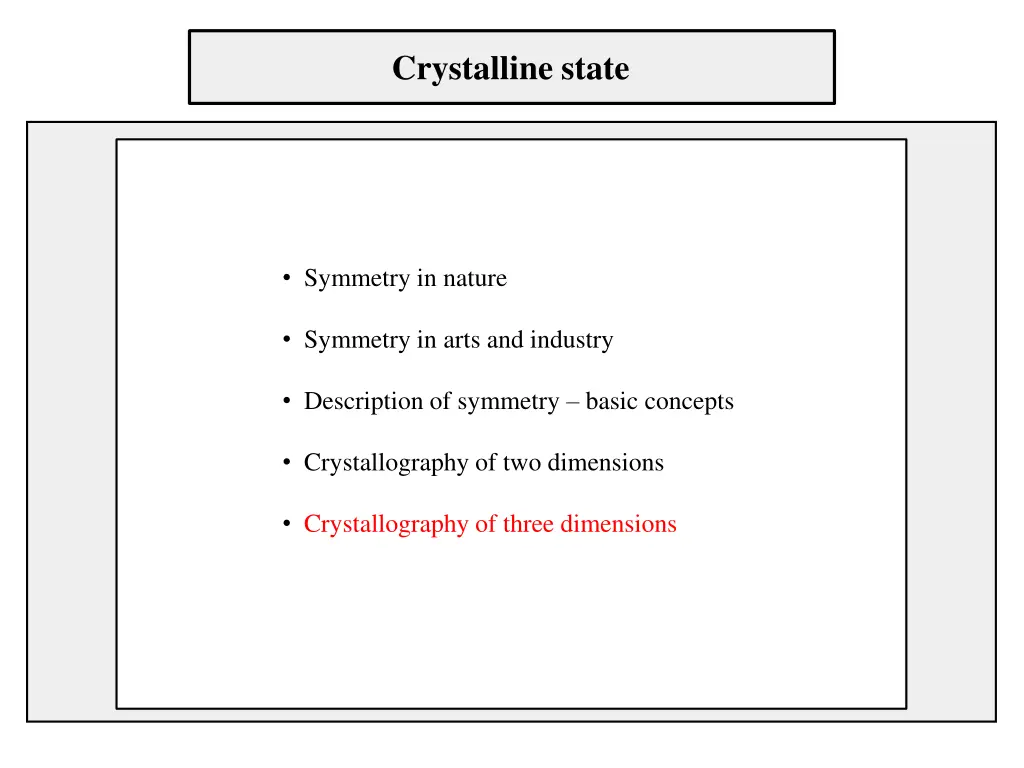
Crystalline state Symmetry in nature Symmetry in arts and industry Description of symmetry basic concepts Crystallography of two dimensions Crystallography of three dimensions
Symmetry elements in 3D Simple symmetry elements point operations Rotation axes Reflection planes Inversion center stred s mernosti 1, 2, 3, 4, 6 m i, Compound symmetry elements point operations Rotoinversions inverzn rota n osi Rotoreflections zrkadlov rota n osi
Rotoinversion (= i)
Fourfold inversion axis new symmetry element
Glide planes in 3D Compound symmetry elements with translations d n a, b, c printed symbol (a b)/4, etc. (a b c)/4 (a b)/2, etc. a/2, b/2, c/2 translation component
Screw axes skrutkov rotan osi Compound symmetry elements with translations Screw axes graphical symbols printed symbols
Screw axes repetition of points
Combination of intersecting axes - every two rotations around intersecting axes can be replaced by one appropriate rotation - the angles and the orientation of the axes are arbitrary - strong limitation for crystal structures - the rotation angles can acquire only the values 0 , 60 , 90 , 120 , 180 - the same holds for the resulting rotation which axis combinations are allowed? there are 35 triplets of axes but only the following combinations are allowed 222, 223, 224, 226, 233, 234
Axial combinations Three axes A , B and C with rotations , and , angles between the axes u, v and w axes w u v C 222 180 180 180 90 90 90 u 223 180 180 120 60 90 90 v 224 180 180 90 45 90 90 B 226 180 180 60 30 90 90 A w 233 180 120 120 54 44' 70 32' 54 44' 234 180 120 90 35 16' 54 44' 45
32 point groups in 3D permissible axes and their combinations 1, 2, 3, 4, 6, 222, 223, 224, 226, 233, 234, combination with mirror planes and inversion center 1
Seven crystal systems The presence (or absence) of rotation axes allows to clasify the crystal structures The characteristic symmetry indicates the minimal symmetry that is always present in each crystal system
Crystal systems and the point groups red possess only rotation axes - enantiomorphic magenta possess a center of inversion centrosymmetric bold referred to as polar
Polar groups Those point groups for which every operation leaves more than one common point unmoved are known as the polar point groups. 1, 2, 3, 4, 6, m, mm2, 3m, 4mm and 6mm Polar direction pol rny smer Direction which is not symmetry equivalent to its opposite direction. Polar direction can only exist in 21 non-centrosymmetric point groups. 20 of them are piezoelectric point groups crystals with this symmetry exhibit piezoelectricity. Exception: group 432 center of symmetry not present, but piezoelectricity cannot occur. Unique direction jedine n smer Direction that is just one and that is not repeated by any symmetry operation. All unique directions are polar directions, but only some polar directions are unique. Unique directions are present only in 8 of 10 polar groups: 2, 3, 4, 6, mm2, 3m, 4mm and 6mm Groups 1 and m are excluded
Stereographic projection How to represent three-dimensional angular relations in plane? Stereographic projection is a quantitative method for presenting three-dimensional orientation relationships between crystallographic planes and directions on a two- dimensional figure.
Point groups in stereographic projection TETRAGONAL SYSTEM
14 Bravais lattices special centering of hexagonal lattice
Hexagonal & rhombohedral indices cr br ar
Cubic & rhombohedral indices br cr ar
Transformation of indices - example LSMO space group R-3c (167) hexagonal indexing conversion to pseudo-cubic lattice and indexing a ~ 0.776 nm hexagonal rhombohedral rhombohedral cubic 012 020 (if a ~ 0.388 then 010) 104 220
Symbols of space groups point group Sch nflies notation Lijk International notation L = lattice capital letter for 3D lattice L P primitive I body centered C centered F face centered R trigonal ijk =symmetry elements of space group for the different symmetry directions
Wyckoff symbols x, y, z coordinates of a point expressed in units a, b, c fractional coordinates frak n s radnice
230 space groups Comprehensive derivation: M. J. Buerger: Elementary Crystallography, MIT Press, 1978, pp. 199-459 Uneven distribution of crystal structures 70% of elements belong to 4 groups face-centered cubic body-centered cubic hexagonal close-packed diamond cubic 60% of organic crystalline compounds have one of six space groups
Examples of structures fcc face-centered cubic four points of space lattice/cell 0,0,0; 1/2, 1/2, 0; 0, 1/2, 1/2; 1/2, 0, 1/2 Al, Cu, Ag, Pd, Pt, Ir bcc body-centered cubic two points of space lattice/cell 0,0,0; 1/2, 1/2, 1/2 Fe, Li, Na, K, Rb, Ba, V, Cr, crystals with the same lattice may have very different structure iron
Examples of structures -manganese 1. 2. x = 0.089; z = 0.278 x = 0.356; z = 0.042 g g c x = 0.356 important!! 1/2 is not 0.5
Examples of structures hcp hexagonal close-packed one points of space lattice/cell 0,0,0 two atoms/point 1/3, 2/3, 1/4; 2/3, 1/3, 3/4 0, 0, 0; Be, Mg, Co, Zn, Zr, Ru 1/3, 2/3, 1/2 diamond structure fcc lattice, four points of space lattice/cell two atoms/point 1/8, 1/8, 1/8; 7/8, 7/8, 7/8 0,0,0; 1/4, 1/4, 1/4 C, Si, Ge GaAs, InP different atoms at two positions
Examples of structures barium titanate BaTiO3 lattice? points/cell? motif? primitive one/cell 5 atoms barium titanium oxygen
How to use the data? 1. X-ray diffraction 2. identification of phases 3. lattice parameters, space group 4. position of atoms 5. calculation of theoretical diffraction pattern 1700 1700 1600 1600 1500 1500 1400 1400 1300 1300 1200 1200 1100 1100 L in (C o u n ts ) L in (C o u n ts ) 1000 1000 900 900 800 800 700 700 600 600 500 500 400 400 300 300 200 200 100 100 0 0 15 20 30 40 50 60 70 80 90 100 15 20 30 40 50 60 70 80 90 100 2-Theta - Scale 2-Theta - Scale ferrit-T39-T2-alfa2_gi-LiF - File: ferrit-T39-T2-alfa2_gi-LiF.raw - Type: 2Th alone - Start: 15.00000 - End: 100.00000 - Step: 0.02000 - Step time: 1. s - Temp.: 25 C (Room) - Time Started: 7 s - 2-Theta: 15.00000 ferrit-T39-T2-alfa2_gi-LiF - File: ferrit-T39-T2-alfa2_gi-LiF.raw - Type: 2Th alone - Start: 15.00000 - End: 100.00000 - Step: 0.02000 - Step time: 1. s - Temp.: 25 C (Room) - Time Started: 7 s - 2-Theta: 15.00000 Operations: Import Operations: Import 00-008-0234 (N) - Nickel Zinc Iron Oxide - (Ni,Zn)Fe2O4/(Ni,Zn)O Fe2O3 - Y: 53.26 % - d x by: 1. - W L: 1.5406 - Cubic - a 8.39900 - b 8.39900 - c 8.39900 - alpha 90.000 - beta 90.000 - gamma 90.000 - Face-centere
Example ZnO output from Data base Int. Tables for Crystallography
Example Ga2O3 output from Data base Int. Tables for Crystallography