Exploring the Application of PDSA Project Model in Research Training Series
Delve into the Society of Metabolic Health Practitioners' Module on the Application of the PDSA Project Model, presented by Dr. Melanie M. Tidman. Learn about the purpose of using a PDSA, mini project design, elements of a PDSA PICOT, and how to create a clinical mini-project using the PDSA format.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
SMHP Research Academy SMHP Research Academy Research Training Series Research Training Series Module Six Module Six
The Society of Metabolic Health Practitioners The Society of Metabolic Health Practitioners Research Training Series Research Training Series Module Six Module Six The Application of the PDSA Project Model The Application of the PDSA Project Model Presented By: Dr. Melanie M. Tidman DHSc, MA, OTR/L, MHP 2
Research Training Video Modules Research Training Video Modules Module One Module One Preparing a Clinical Question for Investigation: What is a PICO(T) Preparing a Clinical Question for Investigation: What is a PICO(T) Module Two Module Two Reference Software and Reference Database Management Reference Software and Reference Database Management Module Three Module Three Study Design and Definition of Variables Study Design and Definition of Variables Module Four Module Four The Role of the IRB and Types of Review The Role of the IRB and Types of Review Module Five Module Five Evaluating the Quality of Research Articles Evaluating the Quality of Research Articles Module Six Module Six The Application of the PDSA Project Model The Application of the PDSA Project Model Module Seven Module Seven Types of Research Articles Types of Research Articles Module Eight Module Eight A Brief Review of R, A Brief Review of R, SPSS and SPSS and Python for Data Analysis Python for Data Analysis Module Nine Module Nine The Search for a Journal and Navigating the Author Guidelines The Search for a Journal and Navigating the Author Guidelines Module Ten Module Ten Preparing the Manuscript Preparing the Manuscript 3
Objectives Objectives Discuss the purpose of using a PDSA Discuss the purpose of using a PDSA Mini Project Design. Mini Project Design. Define the elements of a PDSA PICOT. Define the elements of a PDSA PICOT. Create a Clinical Mini Create a Clinical Mini- -Project using the Project using the PDSA format. PDSA format. 4
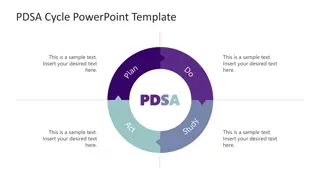
PDSA Project Graphic Description PDSA Project Graphic Description
Example PDSA Project Model: Example PDSA Project Model: Patients with T2D Patients with T2D 6
PDSA Project Model Steps PDSA Project Model Steps Go through PDSA Steps Plan Determine PICO(T) To develop your clinical question Form the blueprint of your investigation. Do Carry out your plan and gather data. Study Analyze your results. Act 7 Adopt, adapt, or abandon the cycle.
The PICO(T) Model The PICO(T) Model P Participants, Patients, Primary Population I The Standard of Care or Common Intervention C Comparison Intervention or New Treatment Intervention/Method O Intended Outcomes (T) Optional Timeframe or Time Period 8
PICO(T) Project Model P Participants, Patients, Primary Population Patients with Type 2 Diabetes 9
PICO(T) Project Model I The Standard of Care or Common Intervention Current ADA Guidelines for Carbohydrate Intake 10
PICO(T) Project Model C Comparison Intervention or New Treatment Intervention/Method A Low Carb Diet with Carbohydrates under 20 grams of Total Carbs Per Day 11
PICO(T) Project Model O Intended Outcomes Reduced HgA1C/HbA1C levels 12
PICO(T) Project Model (T) Optional Timeframe or Time Period In 90 days 13
Plan Plan Project Model PDSA PICOT: PDSA PICOT: P P Patients with Type 2 Diabetes I I Current ADA Guidelines for Carbohydrate Intake C C A Low Carb Diet with Carbohydrates under 20 grams of Total Carbs Per Day O O Reduced HgA1C/HbA1C levels (T) (T) In 90 days 14
Do Do Project Model Parameters: Enroll 2 small groups: could be 5 in each group, age-matched, similar demographics Test HgA1C/HbA1C at baseline Group A: Review the Current ADA guidelines and provide written materials on dietary intake levels of Fats, Proteins and Carbohydrates up to 60% of total daily calories Group B: Review the LCHF Food pyramid and provide written materials on dietary intake of Fats, Proteins, and Carbohydrates, keeping total daily carbs under 20g/day or less than 10% of daily calories. Retest HgA1C/HbA1C in 90 days
Study Study Project Model Results guide future design! Review results and note any trends. Note any barriers or challenges through participant interviews and reports. Note issues with dietary compliance. Note any changes that need to be made to design a larger study including those changes.
Act Act Project Model Re-design variables to be addressed. Written materials versus face-to-face interactions at different points in the 90 days. Possibility of the use of dietary tracking apps for improved compliance. Possibility of daily glucose testing with a Home Glucometer for feedback.
PDSA Model Application PDSA Model Application Plan Use the PICOT format to plan your investigation, identify the population or problem, etc. Do Run the PDSA for the allotted timeframe Study Review any relevant literature or sources, gather resources, read related studies, Review the results, Act Formulate new variables, create another type of method or intervention, and identify challenges or change approaches. 18
Summary Summary The PDSA can: The PDSA can: Be a step towards the design of a future research Be a step towards the design of a future research study on a larger scale. study on a larger scale. Involve a small group with a limited timeframe. Involve a small group with a limited timeframe. Help define the variables, address any issues or Help define the variables, address any issues or challenges with the design, adjust parameters, and challenges with the design, adjust parameters, and provide information on possible trends for further provide information on possible trends for further investigation. investigation. Require less time as it does not involve statistical Require less time as it does not involve statistical analysis with simple data collection. analysis with simple data collection. May qualify for an Exempt or Expedited review 19
References References Agency for Healthcare Quality and Research (2015). Plan-Do-Study-Act Worksheet Directions and Examples. Health Literacy Universal Precautions Toolkit, 3rd Edition: https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html University of Minnesota (2024). Evidence Based Practice: Formulating the Question. https://pressbooks.umn.edu/evidencebasedpractice/chapter/formulatingthequestion/ 20
Acknowledgements Acknowledgements Sincere Thanks to the Society for Metabolic Health Practitioners for their support of this Research Training Series A/V Editing & Tech Support: Bryson Tidman PowerPoint/Graphic Design: Kathryn Tidman 21
SMHP Research Academy SMHP Research Academy Research Training Series Research Training Series Module Six Module Six