Exploring Thermo Datasets in TEdit Interface
Dive into the world of thermo datasets with TEdit, a versatile tool offering multiple ways to view data. Uncover detailed descriptions, activity coefficients, log Ks, and more essential information. Understand the composition of species, basis species for reactions, and redox couples. Discover how to manipulate and analyze data efficiently, all in one comprehensive platform.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
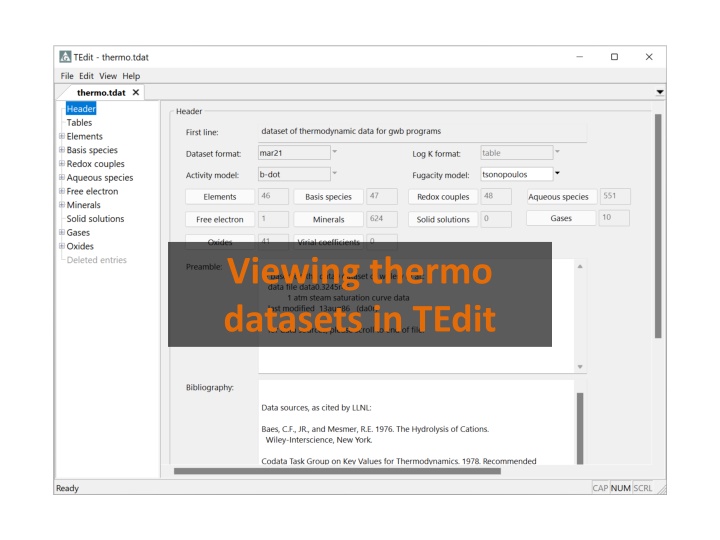
Viewing thermo datasets in TEdit
You can launch TEdit to view thermo datasets in several different ways. GWB s default dataset From TEdit, File Open From GWB apps, File View \thermo.tdat From File Explorer, double-click the file or drag into TEdit
The Header section contains a description of the thermo dataset s content and bibliographic information The B-dot Debye-H ckel equation is used for activity coefficients, and the Tsonopoulos method for fugacity coefficients # of elements, species, etc. in the dataset Literature sources for thermo data
The Tables section contains the principal temperatures at which activity coefficient parameters and log Ks are compiled Data compiled from 0 to 300 C Pressure is by default 1 atm up to 100 C, then follows the steam saturation curve Parameters for calculating activity coefficients
The Elements from which all species in the dataset are composed are defined here Click + to expand list Each entry includes a name, symbol, and molecular weight Click to view the entry
The Basis species from which all reactions are constructed are defined here Each entry includes a name, charge, ion size parameter, and molecular weight Basis species are composed of elements defined in the previous section HCO3 is composed of 1 H, 1 C, and 3 O
The Redox couples are Basis species in a different oxidation state. Entries include a reaction and log K. Species defined in the dataset Negative coefficient for species on the left side of the reaction Reaction might use e , O2(aq), O2(g), H2(aq), or H2(g) Log K for the reaction at the principal temperatures
All other species to be considered are known as Aqueous species In GWB 2021 and newer releases, reactions can be written in terms of any species in the database The reaction should be written without change in oxidation state, if possible
The Free electron is included in datasets considering redox reactions Reaction is balanced in terms of O2(aq), O2(g), H2(aq), or H2(g)
Mineral reactions are defined here Optional information for minerals In GWB 2021 and newer releases, reactions can be written in terms of any species in the database Like the Aqueous species and later sections, reaction should be written without change in oxidation state, if possible
In GWB 2021 and new releases, reactions can be written in terms of any species in the database Gas reactions are defined here CH4(g) is balanced in terms of the Redox species CH4(aq) Parameters for calculating fugacity coefficients. If missing, program assumes ideal behavior
The Oxide components are fictive entries used to describe bulk composition They have no thermodynamic stability and hence there are no entries for log K values
For more details, see the Thermo Datasets chapter in the GWB Reference Manual

![Read⚡ebook✔[PDF] Io After Galileo: A New View of Jupiter's Volcanic Moon (Sprin](/thumb/21612/read-ebook-pdf-io-after-galileo-a-new-view-of-jupiter-s-volcanic-moon-sprin.jpg)
![(❤Read⚡) [✔PDF✔] The Best Travel Guide - San Diego: A Cicerone’s View of To](/thumb/68088/read-pdf-the-best-travel-guide-san-diego-a-cicerone-s-view-of-to.jpg)