Factors Affecting PD-L1/PD-1 Expression: Mechanistic Insights
PD-L1 and PD-1 expression in tumors is regulated by various factors including cytokines, microRNAs, and genetic mechanisms. This article delves into the signaling pathways and mechanisms influencing PD-L1 expression, discussing both constitutive and inducible expression patterns. Understanding these factors is crucial for developing effective immunotherapies targeting the PD-L1/PD-1 axis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
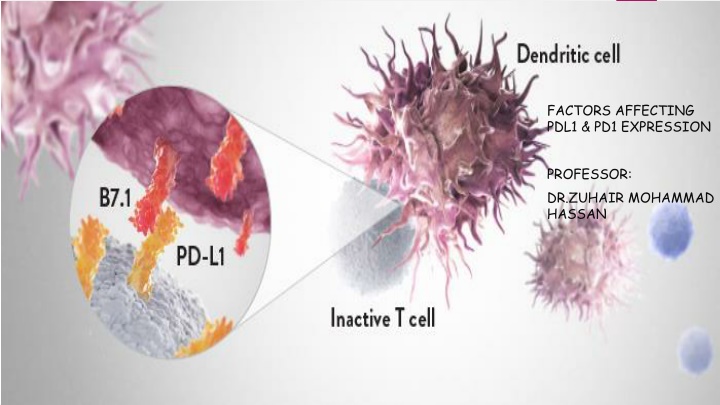
FACTORS AFFECTING PDL1 & PD1 EXPRESSION PROFESSOR: DR.ZUHAIR MOHAMMAD HASSAN
Headlines Introduction Signaling Mechanisms regulating PD-L1 expression The Effect of cytokines on the PD1 & PDL1 expression The effect of some microRnas on the PD1 & PDL1 expression Immunotherapy
Introduction PD-L1, also known as B7-H1, is a type I transmembrane protein, which is expressed in different kinds of tumor cell . PD-L1 can bind to PD-1, which leads to the suppression of lymphocyte activation and apoptosis of lymphocytes PD-L1 can regulate tumor microenvironment or tumor related immune response through suppressing T cell or NK cell mediated immune response. PD-L1expression is regulated by various cytokines, such as IFNg , TGF B, and various microRNAs
Mechanisms regulating PD-L1 expression A tumor can be positive or negative for surface PD-L1 expression through several biological processes, thereby having different clinical significance. These can be categorized as: genetic mechanisms that lead to constitutive PD-L1 expression induced PD-L1 expression by the presence of T cells absence of T cells leading to no reactive PD-L1 expression
Constitutive PD-L1 expression PD-L1 has been reported to be constitutively expressed through the dysregulation of several oncogenic pathways, including : genetic amplification of chromosome 9 PTEN deletions or PI3K/AKT mutations EGFR mutations MYC overexpression
Inducible PD-L1 expression The interferon-inducible expression of PD-L1 is more common than the constitutive expression in most cancer histologies The PD-L1 adaptive expression is a consequence of the presence of tumor antigen specific T cells that recognized the cancer cells leading to the production of interferon- . The physiological induction of PD-L1 by interferon- leads to evasion of a T cell response, termed adaptive immune resistance
Dual Faces of IFNg in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro and Antitumor Immunity IFNg elicits potent antitumor immunity by inducing Th1 polarization, CTL activation, and dendritic cell tumoricidal activity. However, there are significant discrepancies in our understanding of the role of IFNg as an antitumor cytokine
Role of IFNg in physiologic and tumor immunity IFNg plays a pivotal role in systemic and local immunity and is involved in almost all inflammatory responses IFNg is a key cytokine in the polarization of Th1 cells. IFNg secretion by NK cells and dendritic cells (DC) causes the local production of IL12 and there by induces aTh1 response IFNg is essential to the induction of the proliferation of CTL precursors and their differentiation into CTLs
Conflicting Data from Basic and Clinical Research on IFNg Treatment Negative effects of IFNg on tumor inhibition: (SOCS1)-deficient mice spontaneously developed colorectal carcinomas in an IFNg-dependent manner IFNg has been demonstrated to promote papilloma development Mouse mammary adenocarcinomas transfected with the murine IFNg gene give rise to progressive tumors
We propose IFNg-induced programmed cell death 1 ligand 1 (PD-L1) expression as a novel mechanism by which IFNg impairs tumor immunity When tumor cells encounter CTLs in the local environment, they detect them via the high concentration of IFNg secreted from CTLs, which induces PD-L1 expression in preparation for an immune attack.
Use of an ovarian cancer model to investigate the mechanism underlying PD-L1 expression in most of the human and mouse ovarian cancer cells, PD-L1expression was strongly induced by IFNg. Other cytokines, including IL2, IL6, and TGFb, did not induce PD-L1 expression in vitro. we cocultured mouse ovarian cancer cells with mouse CD8+ T cells recovered from the ascites of cancer-inoculated mice or with the supernatants of the ascites fluid. Notably, PD-L1 expression by the cancer cells was strongly induced by coculture with CD8+ T cells but not with the ascetic supernatant, which suggests that direct contact with T cells is necessary for the induction of PD-L1. It is possible that paracrine exposure to the IFNg secreted by T cells induces PD-L1.
Use of an ovarian cancer model to investigate the mechanism underlying PD-L1 expression To test this hypothesis, the IFNg receptor was knocked down in ovarian cancer cells using shRNA, and mice were intra-abdominally inoculated with these cells. The expression of PD-L1 by the IFNg receptor depleted cancer cells was reduced,which indicates that IFNg also mediated PD-L1 expression invivo Consequently , CD8+T-cell infiltration into the tumor site was significantly increased ,and the survival of the mice was significantly improved compared with the mice inoculated with control mouse ovarian cancer cells
transforming growth factor- (TGF-) Because increased TGF- 1production is a hallmark of most TME, we chose to further explore its role in PD-1 expression To assess the effects of cytokines known to alter T cell development, function, and/or proliferation on PD-1 expression: CD3+ T cells isolated from healthy donor peripheral blood mononuclear cells (PBMC) activated them with CD3/ CD28-conjugated beads in the presence of one of 16 cytokines across a range of concentrations The cells were labeled with CFSE to monitor cellular proliferation
Results CD3/ CD28 induces higher PD-1 expression compared to resting CD8+ and CD4+ T cells, confirming TCR and co-stimulation dependent PD-1 expression While most of the cytokines tested had no effect or only a modest effect on PD-1 expression upon T cell activation, major enhancement of PD-1 expression was observed with TGF- 1
TGF-1 did not have any effects on cellular proliferation as measured by CFSE dilution suggesting that enhanced PD-1 expression is not simply due to altered cellular proliferation
Factors affecting the effect of the TGF- To test whether TGF 1-mediated enhancement of PD-1 expression depends on the basal level of PD-1 expression, we isolated na ve T cells and memory T cells from healthy donors The cells were activated with CD3/ CD28-conjugated beads with TGF 1 the effect was more pronounced on na ve T cells than on memory T cells for both CD4 and CD8 subsets
In the absence of CD3/CD28, TGF-1 does not affect the basal levels of PD-1 expression on either na ve or memory T cell subsets. This suggests that TGF- 1 enhancement of PD-1 expression is dependent on T cell activation (TGF- 1) directly enhances antigen-induced PD-1 expression through Smad3 -dependent, Smad2 -independent transcriptional activation in T cells in vitro and in TIL in vivo The PD-1hi subset seen in CD8+ TIL is absent in Smad3 -deficient tumorspecific CD8+ TIL, resulting in enhanced cytokine production by TIL and in draining lymph nodes and of anti-tumor activity
TGF- receptor I (TGF-RI) kinase activity is critical for TGF- -dependent enhancement of PD-1 expression To address the role of TGF- 1 receptor signaling , they used neutralizing antibody (nAb) : TGFB inhibitor TGF- RI kinase inhibitor (SB431542)
Both TGF-1 nAb and SB431542 decreased TGF-1-dependent PD-1 expression , although SB431542 was more effective than TGF- 1 nAb
NFAT Given the critical role of nuclear factor of activated T cell (NFATc1) during TCR dependent PD-1 induction we tested whether TGF- 1-dependent PD-1 expression requires NFATc1 by treating cells with cyclosporine A (CsA) We found that CsA completely abrogated not only TCR-dependent but also TGF- 1 enhanced PD-1 expression suggesting that TGF- 1 requires TCR-induced NFATc1 activity to enhance the PD-1 expression
The Roles of microRNAs in Regulating the Expression of PD-1/PD-L1 Immune Checkpoint With the development of whole genome sequencing technologies, microRNAs have gained more attention as an important new layer of molecular regulation. Recent studies have revealed that altered expression of microRNAs play a pivotal role in immune checkpoint and various cellular processes in cancer
. Current Knowledge on MicroRNAs Involved in PD-1/PD-L1 Regulation microRNA effect on PD1 & PDL1 expression Directly : Some microRNAs have been found to directly target the 3`-UTR of PD-1 or PD-L1 mRNA Indirectly : Some microRNAs may regulate PD-1/PD-L1 indirectly via signaling molecules such as PTEN, STAT , and so on
MicroRNAs That Regulate PD-1/PD-L1 Directly MicroRNAs Regulating PD-1 Expression MiR-28 MiR-138 MiR-4717 Reduction of PD1 expression Inhibition of PD1 expression Reduction of PD1 expression regulating T cell exhaustion
MicroRNAs Regulating PD-L1 Expression miR-15a & miR-16 PD-L1 mRNA dropped in malignant pleural mesothelioma cell line (VMC23) after transfection with miR-15a, miR-15b or miR-16 mimic miR-34a in 44 acute myeloid leukemia (AML) samples , Over-expression of miR-34a in HL-60 and Kasumi-1 cells reduced the expression of PD-L1 miR-138 To examine the effect of miR-138 on endogenous PD-L1 expression, two cell lines with low miR-138 expression, colorectal cancer cell lines (HCT116 and SW620), were transfected with miR-138 mimics. PD-L1 protein levels were decreas
Potential PD-1/PD-L1 Regulatory microRNAs Some emicroRNAs that may affect PD-1/PD-L1expression via regulation of the related signaling pathways such as IFN- / IFNGR / JAK / STAT / PI3K / AKT / MEK / ERK and so on. MicroRNAs Regulating IFN- Expression : miR-181a MicroRNAs Regulating IFNGR Expression: miR-378 MicroRNAs Regulating STAT1 Expression: MiR-150 , MiR-223 MicroRNAs Regulating PTEN Expression : MiR-10a / MiR-19a/ MiR-19b
PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy Several cancers are highly refractory to conventional chemotherapy Check point antibody inhibitors, such as anti-PD-1/PD-L1, are a novel class of inhibitors that function as a tumor suppressing factor via modulation of immune cell-tumor cell interaction These checkpoint blockers are rapidly becoming a highly promising cancer therapeutic approach that yields remarkable antitumor responses with limited side effects
MAbs can: being able to shrink solid tumors suppress advanced tumors and metastasis and overall improve patient survival
PD-1 INHIBITORS APPROVED BY FDA Nivolumab : In the Treatment of: Melanoma HNSCC Renal Cell Carcinoma NSCLC Pembrolizumab: in the treatment of : Head and Neck Squamous Cell Carcinoma (HNSCC)
PD-L1 INHIBITORS APPROVED BY FDA Atezolizumab In the Treatment of : NSCLC Urothelial Carcinoma
PD-1 INHIBITORS FOR COMBINATION THERAPY Considering the safety and better clinical activity of monotherapy the field is moving toward the direction of discovering novel combination therapies Various anti-cancer agents including other check point inhibitors, kinase inhibitors, chemotherapeutics, and targeting agents are used in combination with PD-1 antibody inhibitors
Combination of PD-1 and Other Checkpoint Inhibitors ipilimumab as an anti-CTLA4 Ab , has shown durable anti-tumor activities and prolonged survival in participants with advanced melanoma. ipilimumab, perhaps because of its function in the priming phase of the immune response, it also appears to activate immunity against many types of normal tissues causing severe immune-related adverse events (irAEs) in a substantial minority of patients The combination of ipilimumab and Nivolumab are undergoing human trials for advance melanoma, and nonsmall cell lung carcinoma
Combination of PD-1 and Kinases Inhibitors Mitogen-activated protein kinase (MAPK) is an effective regulator of BRAF mutation, which is responsible for the metastasis of cutaneous melanoma In the clinic, BRAF inhibition has improved metastasis of melanoma The mutation of BRAF has also increased the expression of the PD-1 and PDL-1 molecule and induces potential drug resistance with the involvement of tumor stromal cells The combination of PD1 immunotherapy with a BRAF inhibitor resulted in synergistic anti-tumor response and prominent tumor growth inhibition
There is proof that VEGF suppresses dendritic cell (DC) functions; thus, restoring DC and T-cell activity could improve immune response and anti- tumor activity A phase I clinical study of VEGF inhibitors (bevacizumab) combined with Nivolumab is ongoing for stage III NSCLC patients and unresectable stage III or IV melanoma
Combination of PD-1 inhibitor and Chemotherapeutics PD-1 inhibitors are being treated in combination with antineoplastic agents, such as cisplatin, to achieve a longlasting synergistic anti-cancer effect For example: a phase 3 study is recruiting gastric adenocarcinoma patients for combination of pembrolizumab either with cisplatin or 5-fluorouracil
Recent trends in cancer treatment are moving toward combination immunotherapy, but its success depends on addressing the challenges of choosing the right drug combination, optimizing the dose and schedule of the combination regimen, and managing toxicities and side effects.