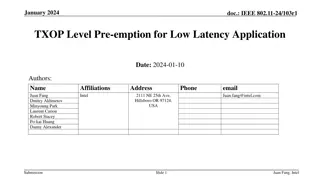
FDA Pre-Submission Meetings for 510(k) Submissions: Key Insights
Learn about the significance of FDA pre-submission meetings for 510(k) submissions, including what they entail, when to request them, and how to prepare effectively. Explore the guidance, meeting requirements, and the scope of topics covered to enhance your regulatory strategy.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Slide 1 of 29 FDA Pre-Submission Meetings for 510(k) Submissions Consultants and RA Experts have used these for years, but they weren t called pre-sub meetings. Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 2 of 29 Agenda What is a pre-sub meeting? Is a pre-sub meeting required for 510(k) submissions? When to request a pre-sub meeting Integrating pre-sub into design plan Preparing for a pre-sub meeting Format and content of a pre-sub request In Person vs. Teleconference What you should never ask during a pre-sub What if you disagree with the FDA? Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 3 of 29 What is a pre-sub meeting? A meeting with the FDA in order to ask questions specific to a planned regulatory submission: 510(k) Application De Novo Application Investigation Device Exemption (IDE) Pre-Market Approval (PMA) Usually a one-time meeting rather than iterative Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 4 of 29 Guidance The Pre-Submission Program and Meetings with FDA Staff Draft guidance issued July 13, 2012 Final guidance issued February 18, 2014 Supersedes Pre-IDE Program: Issues and Answers - Blue Book Memo D99-1, dated March 25, 1999 http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/ guidancedocuments/ucm311176.pdf Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 5 of 29 Not Just for Pre-IDE Pre-sub meetings are not used just for preparing an Investigational Device Exemption (IDE) Submission Other Uses: De Novo Submissions Review of Draft Special Controls Pre-Subs tracked as Q Submissions Meetings Upon Request 75-90 Days from the date of Submission http://www.fda.gov/downloads/training/cdrhlearn/ucm387291.pdf Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 6 of 29 Is a pre-sub meeting required? Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 7 of 29 Submission Logistics 1 eCopy (http://bit.ly/FDA-eCopy) & 1 hardcopy Cover letter Content Identify submission as Pre-sub Meeting Request Sponsor Contact Info Device Name Information specific to Q-Sub type (Should be Pre-Submission ) Address: U.S. Food and Drug Administration Center for Devices and Radiological Health Document Control Center WO66-G609 10903 New Hampshire Avenue Silver Spring, MD 20993-0002 DCC = Document Control Center TLA = Three Letter Acronym Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 8 of 29 When to have a pre-sub? Earliest Time When you have reviewed and approved your design inputs. Latest Time At design freeze. Before a long-term clinical study. Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 9 of 29 Integrating Pre-Sub w/ Design & Risk Plans Risk Control Option Analysis Risk Control Effectiveness Verification Hazard Identification Risk Management Report Risk Assessment Risk Risk / Benefit Analysis Management Plan D R Product Launch 510(k) Concept Phase Feasibility Phase Development Phase Pilot Phase Release Phase Design Transfer DHF Begins Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 10 of 29 Pre-Sub Timeline RTA Process (0-14 days) Does request qualify as a pre-sub? Is submission sufficiently complete? Contact Submitter to schedule date/time of meeting (15-21 days) Submit agenda for meeting allot last 10 minutes to summarizing discussion and action items FDA Provides preliminary feedback 3-days prior to the pre-sub meeting (sometimes meeting is cancelled) FDA Provides feedback (75-90 days) Sponsor provides draft minutes to DCC (15 days) FDA reviews/edits draft meeting minutes (30 days) May present courtesy copy to reviewer of final meeting minutes. Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 11 of 29 Basics of a Pre-sub Provide several options for dates to remain flexible Agenda should limit presentation of information to approximately 1/3 the allotted time for meeting Clear and concise background information Specific questions for FDA If for a clinical study (NSR, IDE or OUS), then entire protocol may be submitted Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 12 of 29 General Content of a Pre-sub Cover letter Table of contents Device description Proposed Intended/Indications for Use Previous discussions or submissions Overview of product development Specific questions Method for feedback in-person meeting a teleconference Fax email Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 13 of 29 510(k) Content for Pre-Sub Proposed predicate device(s) Trade name & model number 510(k) number Classification of the predicate device Comparison of predicate(s) with subject device Indications for Use Technological characteristics Performance testing Performance Testing Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 14 of 29 Proposed Test Plan Details objective or purpose of the test explanation of the sample size and statistical methods summary of the test methodology (if you are following a recognized standard, include the name of the standard and year of publication) explanation of study endpoints explanation of study acceptance criteria Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 15 of 29 Do not include test results & data Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 16 of 29 If requesting in-person or tcon three (3) or more preferred dates and times when you are available to meet using the guidelines in Table 1 above for scheduling; the planned attendees, including each attendee s position, or title, and affiliation. If you have not yet identified all of your attendees, you should indicate the type of subject matter experts you plan to invite so that we can ensure appropriate FDA experts are in attendance. Please note foreign visitors meeting in an FDA facility require advanced security clearance. See Section IV. B. Security Screening below for additional information on how to request security clearance for Foreign Nationals; and a list of any audiovisual equipment you will need, such as conference phone or LCD projector. Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 17 of 29 Pre-Sub Questions Is justification of no carcinogenicity testing acceptable? Does FDA agree with worst-case rationale? Is software moderate level of concern ? Is planned approach for human factors acceptable? Are there concerns of predicate selected? Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 18 of 29 IVD-Specific Considerations Request a pre-sub before conducting clinical, nonclinical, or analytical studies or submitting a marketing application for a new IVD that: Is a multiplex device capable of simultaneously testing a large number of analytes Contains a new technology Has a new intended use Includes a new analyte Presents new clinical questions Presents complex data/statistical questions Uses a predicate or reference method that is unclear or uncertain Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 19 of 29 Preparing for a Pre-Sub Do your homework 1st. Prepare documentation early and submit with Pre-Sub meeting request including proposed agenda Practice your questions and responses to potential questions by role playing Submit focused questions in advance Develop the agenda based on these questions Bring the right experts to execute your objectives Have an expert present that can kick you . If you are visiting the FDA in-person, be sure to arrive 30 minutes early. FDA will greet you 10 minutes prior. You will need ID for in-person meetings. Have a dedicated scribe to take notes. Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 20 of 29 Clinical Study: Agreement on Study Size Never ask how many patient subjects Always suggest a study design instead Present Clinical Synopsis Or a Clinical Protocol Include non-clinical data Include similar clinical studies with the same device, but a different indication http://medicaldeviceacademy.com/clinical-procedure/ http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm? CFRPart=812 Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 21 of 29 De Novo Data Requirements Generally, isolated case reports, random experience, reports lacking sufficient details to permit scientific evaluation, and unsubstantiated opinions are not regarded as valid scientific evidence to show safety or effectiveness. However, such information may be considered in identifying a device that has questionable safety and effectiveness. IDE not required or foreign clinical studies and studies of non-significant risk (NSR) Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 22 of 29 De Novo Classification? What makes the device a Class I? What makes the device a Class II? What makes the device Class I or Class II exempt? 274 Device Classifications are Class II with 510(k) exemption http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/315.cfm Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 23 of 29 What not to do for Pre-Sub Do not request a meeting just to give the FDA info Do not ask the FDA to help suggest performance bench testing or animal studies Do not ask the FDA to help design a clinical study Do not ask questions that can be directed to the reviewer Do not ask for classification decision 513(g) Do not ask for designation of jurisdiction (RFD) Do not ask for determination on a submission Do not ask for agreement on clinical data requirements Do not ask if data is acceptable Do not bother if you already did your testing Rob Packard, President http://medicaldeviceacademy.com/fda-device-classification/ www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 24 of 29 Non-Binding Feedback FDA intends to stand behind our feedback unless: Information in subsequent submission is not consistent with pre-sub (e.g., change in proposed indication for use or device design) Data in the subsequent submission raise important new issues related to safety and effectiveness (e.g., a study is conducted as recommended by FDA, but results raise new safety concerns) Feedback given previously does not adequately address important new issues materially relevant to a determination of safety or effectiveness that have emerged since the time of the pre-sub (e.g., new alternative therapies/diagnostics have emerged since discussion of the clinical protocol making the previously recommended study design unethical) Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 25 of 29 Meeting Minutes A summary not a transcript No responses to FDA feedback Follow-up requests Submit a Q-Sub Supplement instead Submit draft meeting minutes to DCC FDA edited version becomes final within 15 days, unless sponsor submits minutes disagreement amendment not a disagreement with feedback, but a disagreement with what was said Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 26 of 29 Disagreement with FDA Do not argue, because you don t have to agree with the agency. Summarize action items at the end and ask for clarification. Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 27 of 29 Risk / Benefit Ratio Are there any risks to having a pre-sub meeting? What are the benefits of a pre-sub meeting? Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 28 of 29 Q & A rob@13485cert.com Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com
Slide 29 of 29 Need a quotation for a 510(k) Submission? rob13485 rob@13485cert.com +1.802.281.4381 Rob Packard Rob Packard, President www.MedicalDeviceAcademy.com www.MedicalDeviceAcademy.com rob@13485cert.com




![ANC Women's League Oral Submission on Expropriation Bill [B23.2020]](/thumb/136076/anc-women-s-league-oral-submission-on-expropriation-bill-b23-2020.jpg)