FORMULAS to NAMES IONIC and MOLECULAR
Test your knowledge of naming ionic and molecular compounds with 50 chances to practice matching formulas to proper or stock names. Challenge yourself to accurately identify whether the compound is ionic or molecular based on the elements involved. Learn the rules for naming compounds and improve your skills before the next assessment.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
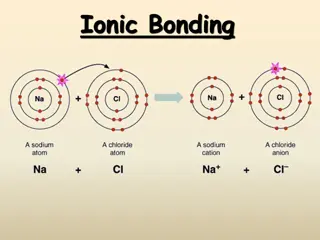
FORMULAS to NAMES IONIC and MOLECULAR Here are 50 chances for you to look at a formula and determine the proper or stock name of the compound. You should first decide if it s IONIC or MOLECULAR. You can tell if the 1st atom or part is a METAL, it s ionic. If the 1st atom is a NONMETAL, then it s is molecular. The only exception is AMMONIUM, which is NH4+1 Ammonium is a polyatomic cation, it is ionic.
Grade yourself harshly: its right or its wrong. Almost right is all wrong. Fix your errors, you are making yourself a key - for home practice. Do this again before Thursday! Learn the rules for naming compounds (one page in the BASICS).
1 MgO What is the name of this compound?
1 MgO Magnesium Oxide Mg is a metal, this is ionic Mg+2 O-2 2:2 ratio becomes 1:1
2 TaF5 What is the name of this compound?
2 TaF5 Tantalum fluoride (no RN, only 1 kind of cation) Ta is a metal, this is ionic Ta+5 F-1 1:5 ratio
3 SrCl2 What is the name of this compound?
3 SrCl2 Strontium chloride Sr is a metal, this is ionic Sr+2 Cl-1 1:2 ratio
4 NiBr3 What is the name of this compound?
4 NiBr3 Nickel III Bromide Ni is a transitional metal, this is ionic Ni+3 Cl-1 1:3 ratio
5 NF3 What is the name of this compound?
5 NF3 Nitrogen trifluoride N is a nonmetal, this is molecular Use the prefix naming system 1:3 ratio
6 TiO2 What is the name of this compound?
6 TiO2 Titanium IV oxide Ti is a transitional metal, this is ionic Ti+4 O-2 The 2:4 ratio 1:2
7 NO2 What is the name of this compound?
7 NO2 Nitrogen dioxide N is a nonmetal, this is molecular Use the prefix naming system 1:2 ratio
8 Ti2O3 What is the name of this compound?
8 Ti2O3 Titanium III oxide Ti is a transitional metal, this is ionic Ti+3 O-2 2:3 ratio
9 TiO What is the name of this compound?
9 TiO Titanium II oxide Ti is a transitional metal, this is ionic Ti+2 O-2 1:1 ratio
10 AlN What is the name of this compound?
10 AlN Aluminum nitride Al is a metal, this is ionic Al+3 N-3 The 3:3 ratio 1:1 ratio
11 PCl3 What is the name of this compound?
11 PCl3 Phosphorous trichloride P is a nonmetal, this is molecular Use the prefix naming system 1:3 ratio
12 BF3 What is the name of this compound?
12 BF3 Boron trifluoride (rhymes with ) B is a nonmetal, this is molecular Use the prefix naming system 1:3 ratio
CF4 Carbon tetrafluoride C is a nonmetal, this is molecular Use the prefix naming system 1:4 ratio
14 N2O3 What is the name of this compound?
14 N2O3 Dinitrogen trioxide N is a nonmetal, this is molecular Use the prefix naming system 2:3 ratio
15 OF2 What is the name of this compound?
15 OF2 Oxygen difluoride O is a nonmetal, this is molecular Use the prefix naming system 1:2 ratio
16 SO3 What is the name of this compound?
16 SO3 Sulfur trioxide S is a nonmetal, this is molecular Use the prefix naming system 1:3 ratio
17 SiO2 What is the name of this compound?
17 SiO2 Silicon dioxide Si is a nonmetal, this is molecular Use the prefix naming system 1:2 ratio
18 SiF4 What is the name of this compound?
18 SiF4 Silicon tetrafluoride Si is a nonmetal, this is molecular Use the prefix naming system 1:4 ratio
19 H2S What is the name of this compound?
19 H2S Dihydrogen monosulfide (this is stinky gas!) H is a nonmetal, this is molecular Use the prefix naming system 2:1 ratio
20 AsBr5 What is the name of this compound?
20 AsBr5 Arsenic pentabromide As is a nonmetal, this is molecular Use the prefix naming system 1:5 ratio
21 SeO3 What is the name of this compound?
21 SeO3 Selenium trioxide Se is a nonmetal, this is molecular Use the prefix naming system 1:3 ratio
22 PI3 What is the name of this compound?
22 PI3 Phosphorous triiodide P is a nonmetal, this is molecular Use the prefix naming system 1:3 ratio
23 FCl What is the name of this compound?
23 FCl Fluorine monochloride F is a nonmetal, this is molecular Use the prefix naming system 1:1 ratio
24 HBr What is the name of this compound?
24 HBr Hydrogen monobromide H is a nonmetal, this is molecular Use the prefix naming system 1:1 ratio