Fuel Cell Using Potassium Hydroxide Solution
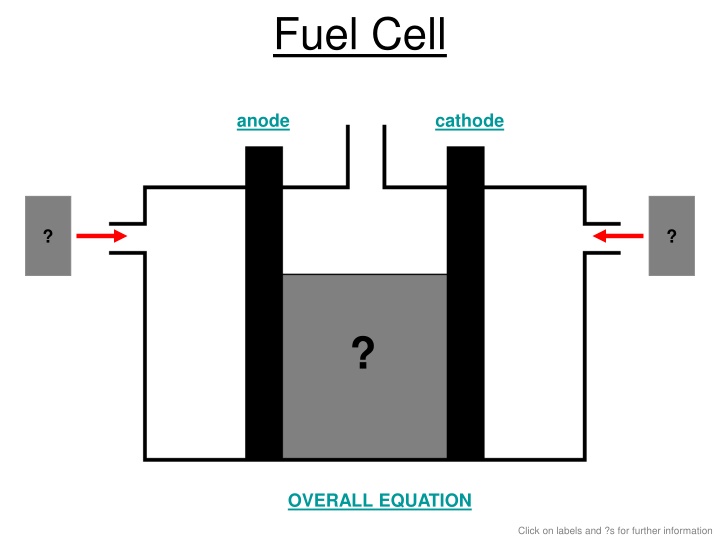
Fuel cells are devices that convert chemical energy into electrical energy through redox reactions. In this specific fuel cell system, the anode consists of porous carbon with nickel serving as a catalyst, where hydrogen gas and hydroxide ions are oxidized to produce water and electrons. The cathode, also made of porous carbon with nickel and nickel oxide acting as catalysts, facilitates the reduction of oxygen gas and water to hydroxide ions and electrons. The overall equation for this fuel cell process results in the production of water with an efficiency of around 70%.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Fuel Cell anode cathode O2 H2 ? ? Potassium Hydroxide Solution ? OVERALL EQUATION Click on labels and ?s for further information
ANODE POROUS CARBON WITH NICKEL (CATALYST) 2H2(g) +4OH-(aq) 4H2O (l) + 4e- Half Equation
CATHODE POROUS CARBON WITH NICKEL & NICKEL OXIDE (CATALYST) O2(g) + 2H2O (l) + 4e- 4OH- (aq) Half Equation
OVERALL EQUATION 2H2(g) + O2(g) 2H2O (aq) about 70% efficiency