Fuel Poverty in the UK
Fuel poverty in the UK is a pressing issue affecting millions of households, where a significant portion of income is spent on heating to an acceptable standard due to poor energy efficiency, low incomes, and high fuel prices. The prevalence of fuel poverty has been steadily increasing, damaging the quality of life for many individuals and families.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Understanding LC-FAODs Rare metabolic disorders arising from genetic variants defective or absent enzymes required for the oxidation of LCFAs1 Impaired fatty acid conversion hinders energy production, especially during fasting or high energy demand2 Toxic fatty acids and metabolites accumulate, leading to organ dysfunction3 LCFAs, long-chain fatty acids; LC-FAODs, long-chain fatty acid oxidation disorders 1. Vockley J, et al. Mitochondrial Fatty Acid Oxidation Defects. 2017; pp. 125-144. 2. El-Gharbawy A, et al. Pediatr Clin North Am. 2018;65:317-335. 3. Vockley J. Am J Manag Care. 2020;26(7 Suppl):S147-S154.
Understanding LC-FAODs LC-FAODs are inherited in an autosomal recessive manner1 1. Vockley J, et al. Mitochondrial Fatty Acid Oxidation Defects. 2017; pp. 125-144. Image reproduced without modification under Creative Commons License CC BY-SA 3.0 . Source: Autosomal recessive inheritance [illustration]. Wikimedia Commons. Published January 20, 2020. https://commons.wikimedia.org/wiki/File:Autosomal_recessive_-_en.svg. Under the terms of
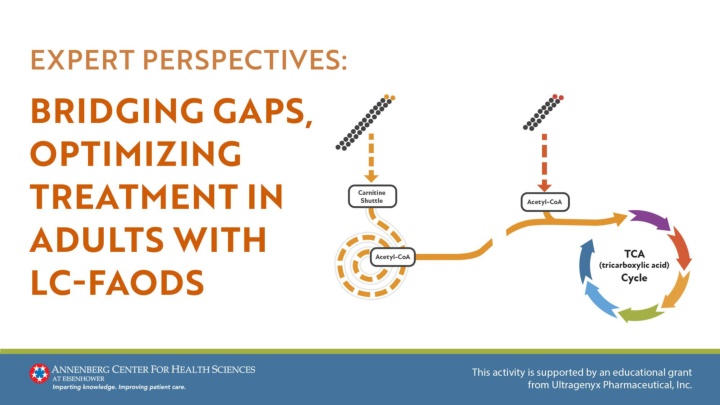
LC-FAODs Are Caused by Enzyme Deficiencies in the Carnitine Shuttle or Long-Chain -oxidation1,2 Step 1: LCFAs are transported into the mitochondria through the carnitine shuttle (MCFAs): Step 2: LC fatty-acyl-coAs are metabolized by long- chain specific enzymes in the -oxidation spiral4 X Once inside the mitochondria, these MCFAs are metabolized into different TCA cycle substrates depending on their even- or odd-chain characteristics Step 3: Acetyl-CoA is produced and can either5,6: 1. Be metabolized to ketones in the liver, or 2. Enter the TCA cycle to generate ATP through oxidative phosphorylation X Step 4: Reducing equivalents from reactions 1 and 3 enter the electron transport chain to generate ATP4 Acetyl-CoA, acetyl coenzyme A; ATP, adenosine triphosphate; CACT, carnitine- acylcarnitine translocase deficiency; CPT 1, carnitine palmitoyltransferase I; CPT II, carnitine palmitoyltransferase II; LCFA, long-chain fatty acid; LCHAD, long-chain L-3 hydroxyacyl-CoA dehydrogenase deficiency; TCA, tricarboxylic acid; TFP, trifunctional protein deficiency; VLCAD, very-long-chain acyl-CoA dehydrogenase deficiency. 1. Wanders RJ, et al. J Inherit Metab Dis. 2010;33(5):479-494. 2. Vockley J, et al. Mol Genet Metab. 2017;120(4):370-377. 3. Tamas C, et al. Int J Mol Sci. 2024;25(10):5482. 4. Vockley J. Am J Manag Care. 2020;26(7 Suppl):S147-S154. 5. Knottnerus SJG, et al. Rev Endocr Metab Disord. 2018;19(1):93-106. 6. Wanders RJ, et al. J Inherit Metab Dis. 2010;33(5):479-494.
Metabolic Consequences of LCFA Enzyme Deficiencies TCA Cycle Imbalance1,3,4 Impaired Long-Chain Fatty Acid Metabolism1,2 Reduced Energy Production1,5-8 Toxic Metabolites ATP Production Gluconeogenesis TCA Cycle Substrates Ketogenesis Lipogenesis 1. Knottnerus SJG, et al. Rev Endocr Metab Disord. 2018;19(1):93-106. 2. Longo N, et al. Am J Med Genet C Semin Med Genet. 2006;142C(2):77-85. 3. Brunengraber H, et al. J Inherit Metab Dis. 2006;29(2-3):327-331. 4. Janeiro P, et al. Eur J Pediatr. 2019;178(3):387- 394. 5. Wajner M, et al. Biosci Rep. 2015;36(1):e00281. 6. Owen OE, et al. J Biol Chem. 2002;277(34):30409-30412. 7. Roe CR, et al. Mol Genet Metab. 2015;116(4):260-268. 8. Houten SM, et al. J Inherit Metab Dis. 2010;33(5):469-477.
Newborn Screening of LC-FAOD NBS for LC-FAOD now conducted in all US states1 Screening typically occurs at 24 48 h of age2 Detects most cases before symptoms1 Early Diagnosis Follow- Up Testing Acylcarnitine profiles can normalize after initial screening2 In states with 2 NBS, a normal second screen does not rule out LC-FAOD Definitive diagnosis may require molecular/enzymatic testing2 NBS, newborn screening 1. Wilcken B. J Inherit Metab Dis. 2010;33:501 506. 2. Baker JJ, et al. touchREV Endocrinol. 2021;17(2):108-111.
Newborn Screening of LC-FAOD Normal NBS does not fully exclude LC- FAOD (especially mild forms)1 Further testing is required if symptoms develop NBS has only been in place for 20 25 y in many states, meaning some adults may not have been screened 1. Baker JJ, et al. touchREV Endocrinol. 2021;17(2):108-111.
A Few Facts About LC-FAODs Approximately 100 newborns in US are diagnosed each year through NBS1 Incidence: 0.9 15.2 per 100,000 births2 Prevalence (US): 2000 35002 1.94 hospitalizations/y for a mean of 17.55 d/y3 1. Vockley J. Am J Manag Care. 2020;26(7 Suppl):S147-S154. 2. Marsden D, et al. Genet Med. 2021;23:816 829. 3. Vockley J, et al. Mol Genet Metab. 2015;116(1-2):53-60.
Disrupted Energy Balance Triggers Acute and Chronic Symptoms Spontaneous and unpredictable Potential long-lasting effects Acute episodes1-3 Can begin h after birth or in adulthood Evolving symptoms over time Symptom onset2,4,5 Episodic, can require hospitalization or emergency care3,5,6 High variability, even among family members7 Symptom nature 1. Wajner M, et al. Biosci Rep. 2015;36(1):e00281. 2. Knottnerus SJG, et al. Rev Endocr Metab Disord. 2018;19(1):93-106. 3. Saudubray JM, et al. J Inherit Metab Dis. 1999;22(4):488-502. 4. Spiekerkoetter U. J Inherit Metab Dis. 2010;33(5):527-532. 5. Vockley J, et al. [published correction appears in Mol Genet Metab. 2015;116(3):221]. Mol Genet Metab. 2015;116(1-2):53-60. 6. Shekhawat PS, et al. Pediatr Res. 2005;57(5 Pt 2):78R-86R. 7. Merritt JL 2nd, et al. Rev Endocr Metab Disord. 2020;21(4):479-493.
LC-FAODs Present as a Spectrum of Symptoms Across Ages Newborn Adulthood Childhood/Adolescence Hypoglycemia (+/- hyperammonemia)/liver dysfunction1 Muscle weakness/rhabdomyolysis1 Heart muscle damage (cardiomyopathy)2,3 1. Vockley J. Am J Manag Care. 2020;26(7 Suppl):S147-S154. 2. Merritt JL 2nd, et al. Rev Endocr Metab Disord. 2020;21(4):479-493. 3. Knottnerus SJG, et al. Rev Endocr Metab Disord. 2018;19(1):93-106.
Additional Signs and Symptoms Peripheral neuropathy* Gastrointestinal symptoms Cognitive issues Retinopathy* Secondary to hypoglycemia, hypoxia, or hyperammonemia Decreases in color, low-light, and central vision Weakness, numbness, and pain Nausea, lack of appetite *Specific for trifunctional protein and long-chain 3-hydroxyacyl-CoA dehydrogenase disorders Merritt JL 2nd, et al. Rev Endocr Metab Disord. 2020;21(4):479-493.
Historical Treatment Primarily Focuses on Nutritional and Symptomatic Management Dietary Modifications MCT Supplementation MCT, medium-chain triglyceride
Historical Treatment Primarily Focuses on Nutritional and Symptomatic Management Dietary modifications Prevent prolonged fasting to reduce the risk of metabolic crises1 Limit long-chain fats to 10% 35% of total energy intake, depending on disease severity2 Mild-to-moderate LC-FAOD: Calories split 50/50 between MCT and long-chain fats1 Severe LC-FAOD: Ratio of 2:1 MCT to long-chain fats1 1. Baker JJ, et al. touchREV Endocrinol. 2021;17(2):108-111. 2. Rohr F. Nutrition Management of Fatty Acid Oxidation Disorders. 2022; pp. 325-335.
Historical Treatment Primarily Focuses on Nutritional and Symptomatic Management MCT supplementation MCTs bypass the defective LCFA metabolism Diffuse across the mitochondrial membrane without needing carnitine transport1 Depend upon the medium-chain acyl-CoA dehydrogenase and short-chain acyl- CoA dehydrogenase enzymes, which are unaffected in LC-FAOD2 MCT doses + long chain fat rarely exceed 35% of total energy intake for adults3 1. Marsden D, et al. Genet Med. 2021;23(5):816-829. 2. Rohr F, Nutrition Management of Fatty Acid Oxidation Disorders. 2022; pp. 325-335.
Triheptanoin Is FDA Approved for the Treatment of LC-FAOD Triheptanoin is a medium-chain triglyceride indicated as a source of calories and fatty acids for the treatment of adult and pediatric patients with molecularly confirmed LC-FAOD Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023.
Triheptanoin Provides an Alternative Energy Source That Bypasses the Metabolic Block in LC-FAODs C7: Triheptanoin* C8: Trioctanoin/MCT Acetyl-CoA + Propionyl-CoA Methylmalonyl-CoA *Enhances TCA cycle efficiency, improving ATP production, gluconeogenesis, and protein synthesis Vockley J, et al. J Inherit Metab Dis. 2023;46(5):943-955.
Clinical Study: Triheptanoin vs Trioctanoin Objective: Triheptanoin (C7) vs trioctanoin (C8) on energy metabolism, cardiac function, and exercise tolerance in patients with LC-FAODs Design: Double-blinded, randomized controlled trial Participants: 32 subjects with LC-FAODs (CPT-2, VLCAD, TFP, or LCHAD deficiencies) Intervention: 4-mo diet containing 20% of total daily energy from either C7 or C8 Gillingham MB, et al. J Inherit Metab Dis. 2017;40(6):831-843.
Triheptanoin vs Trioctanoin (continued) Results Left ventricular (LV) ejection fraction increased by 7.4% (P=0.046) in the C7 group LV wall mass decreased by 8% in the C7 group and increased by 15% in the C8 group, with a significant difference in relative change between the groups (95% CI: 64.8 99.0%; P=0.041) Maximum heart rate was 6.98 beats per minute lower in the C7 group during exercise (P=0.040) No significant differences in total energy expenditure, phosphocreatine recovery, or body composition Overall, patients had better heart function after 4 wks on C7 vs C8 Gillingham MB, et al. J Inherit Metab Dis. 2017;40(6):831-843.
Triheptanoin vs Trioctanoin (continued) Safety Data Seven hospitalizations for acute rhabdomyolysis in both C7 and C8 groups Most adverse events were mild, with no significant differences between groups Common adverse events: intermittent muscle pain, fatigue, and elevated creatine kinase levels No significant difference in GI adverse events between groups Gillingham MB, et al. J Inherit Metab Dis. 2017;40(6):831-843.
Clinical Study: Safety and Efficacy of Triheptanoin Objective: Assess the safety and efficacy of triheptanoin in patients with LC-FAOD with ongoing significant musculoskeletal, cardiac, and/or hepatic events despite standard treatment Design: Single-arm, open-label, phase 2 study (CL201) Participants: Patients with CPT-II, VLCAD, LCHAD, or TFP deficiencies and significant symptoms despite stable treatment Intervention: After 4 wks, patients switched from MCT oil (if applicable) to triheptanoin, titrated to 25% 35% of DCI DCI, daily caloric intake Vockley J, et al. Mol Genet Metab. 2017;120(4):370-377.
Safety and Efficacy of Triheptanoin (continued) Results 12MWT: 28% improvement in distance at wk 18 Exercise tolerance: 60% increase in watts generated at wk 24 HR-QoL: Significant improvement in adult SF-12v2 physical and mental scores; no change in pediatric SF-10 scores 12MWT, 12-minute walk test; HR-QoL, Health-related quality of life Vockley J, et al. Mol Genet Metab. 2017;120(4):370-377.
Safety and Efficacy of Triheptanoin (continued) Safety Data Adverse events: 62% experienced treatment-related adverse events, mainly mild-to-moderate GI issues (55%) One serious event (gastroenteritis) One discontinuation due to moderate diarrhea Vockley J, et al. Mol Genet Metab. 2017;120(4):370-377.
Clinical Study: Long-Term Safety and Efficacy of Triheptanoin Objective: Evaluate the long-term safety and efficacy of triheptanoin in reducing the rate of MCEs in patients with LC- FAODs Design: Open-label, long-term, single-arm extension study (CL202) Participants: Patients age 6 mos with confirmed diagnoses of LC- FAODs (CPT-I, CPT-II, CACT, VLCAD, LCHAD, or TFP deficiencies) Intervention: Patients were switched from MCT oil to triheptanoin, titrated to 25% 35% of daily caloric intake, and treated for up to 7 y MCE, major clinical events (hypoglycemia, cardiomyopathy, or rhabdomyolysis) Vockley J, et al. J Inherit Metab Dis. 2023;46(5):943-955.
Long-Term Safety and Efficacy of Triheptanoin (continued) Results Cohort Baseline MCE Rate* End of Treatment MCE Rate MCE Reduction Hospitalization Reduction Mean Treatment Duration Triheptanoin- na ve 2.00 events/patient/y 0.28 events/patient/y 86% 85% 27.4 19.9 mos CL201 rollover 1.76 events/patient/y* 1.00 events/patient/y 43% 47% 46.9 13.6 mos IST/EAP N/A 1.40 events/patient/y N/A N/A 49.6 21.4 mos *Baseline MCE rate measured before the introduction of C7 and prior to the CL201 study Vockley J, et al. J Inherit Metab Dis. 2023;46(5):943-955.
Long-Term Safety and Efficacy of Triheptanoin (continued) Safety Data Adverse events: 97% of patients reported at least 1 TEAE 68.1% of patients experienced treatment-related AEs, primarily of mild-to-moderate severity 7 serious treatment-related AEs occurred in 5 patients, all of which resolved TEAE, treatment-emergent adverse event Vockley J, et al. J Inherit Metab Dis. 2023;46(5):943-955.
Recommended Dosage of Triheptanoin Calculate based on patient s daily caloric intake Substitute triheptanoin for existing MCT products as it is added to treatment regimen Target dose: Up to 35% of DCI (usually lower for older patients) Dosage Frequency: Divide into at least4 doses with meals/snacks Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023.
Initiation and Titration of Triheptanoin Start at 10% DCI Increase to a goal of up to 35% over 2 3 wks Dose is increased by 5% every 2 3 d New patients Switching from MCT Begin at last tolerated MCT dose, increase by 5% DCI every 2 3 d Use smaller, frequent doses if needed; adjust for GI symptoms Four doses are recommended, but sometimes more Tolerability Maintain at the highest tolerated dose if goal DCI (35%) is not achieved Maximum dose Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023.
Administration Considerations Triheptanoin can be mixed with soft foods or liquids,* such as: Plain or sweetened fat-free yogurt Fat-free milk or cottage cheese Whole grain hot cereal Fat-free pudding Smoothies Applesauce (or similar semi-liquid foods) *Low-fat products rather than fat-free may be appropriate for some patients with LC-FAOD Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023.
Metabolic Requirements and Nutritional Planning Calculate based on individual patient factors* Patients with LC-FAODs can have lower resting and total energy expenditures Modify traditional energy calculations to avoid overestimation Estimated Energy Requirements *For patients known to the clinic, their typical energy intake is often available and can serve as their DCI DeLany JP, et al. Mol Genet Metab. 2023;138(3):107519.
Metabolic Requirements and Nutritional Planning Carbohydrates Ensure adequate intake to prevent hypoglycemia and support energy needs2 Fats Include MCTs (traditional or triheptanoin) to bypass metabolic defects2,3 Proteins Provide sufficient protein to support growth and repair while helping maintain lean body mass2 Macronutrient distribution1 1. DeLany JP, et al. Mol Genet Metab. 2023;138(3):107519. 2. Gillingham MB, et al. J Inherit Metab Dis. 2019;42(5):857-869. 3. Karall D, et al. Orphanet J Rare Dis. 2015;10:21.
Individualized Dosing and Nutritional Planning Adjust triheptanoin dosage based on patient response and tolerability1 Consider using lower doses than FDA-approved, if necessary, based on clinical judgment and patient-specific factors Individualized dosing Tailored approach Customize macronutrient ratios based on patient-specific metabolic needs and response2,3 Regular monitoring Continuously assess metabolic stability and clinical outcomes2,4 Modify dosing and nutritional plans as needed, based on ongoing evaluations and patient feedback1 1. Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023. 2. Vockley J. Am J Manag Care. 2020;26(7 Suppl):S147-S154. 3. Pe a-Quintana L, et al. Nutrients. 2024;16(16):2707. 4. Baker JJ, et al. touchREV Endocrinol. 2021;17(2):108-111.
Triheptanoin vs Traditional MCTs Dosing Differences2* Metabolic Benefits1 Side Effects3 Triheptanoin has a similar side effect profile to standard MCTs, commonly causing diarrhea, abdominal pain/discomfort, and vomiting C8 MCT oil supplies acetyl CoA only, lacking the additional propionyl CoA provided by triheptanoin Traditional C8 MCT Oil: 15% 25% of DCI Triheptanoin: 25% 35%of DCI *Dosing varies with age and disease severity; C7 doses studied in those with severe LC-FAOD 1. Baker JJ, et al. touchREV Endocrinol. 2021;17(2):108-111. 2. Vockley J, et al. Clin Nutr ESPEN. 2021;41:293-298. 3. Vockley J, et al. J Inherit Metab Dis. 2021b;44(1):253-263.
Clinical Case 1 A 28-yo female, diagnosed with late-onset very long-chain acyl-CoA dehydrogenase deficiency at age 12 y, had been stable for some time with MCT oil supplementation at 35 mL/d. Recently, she has experienced increased fatigue and weakness with mild exertion, alongside episodes of rhabdomyolysis at ages 16 y and 22 y. Laboratory tests reveal elevated serum creatine kinase (8,000 U/L), with normal renal function. Due to these persistent symptoms, she has now started triheptanoin therapy. Her weight is 60 kg, and her total DCI is calculated to be 2300 kcal. What would be the appropriate initial TDD and maintenance dosage of triheptanoin for this patient, based on the recommended dosing guidelines? TDD, total daily dose
Answer: Clinical Case 1 Based on her DCI, the recommended maintenance dose of triheptanoin for this patient is 69.3 to 97 mL/d, with an initial TDD of around 35 mL/d due to her prior MCT oil intake* 1. Since the patient is taking 35 mL of MCT oil, she will switch to an equivalent 35 mL dose of triheptanoin (divided into 4 or more doses throughout the day) 2. Determine the calories in 35 mL triheptanoin 35 mL triheptanoin x 8.3 kcal/mL = 290 kcals 3. Triheptanoin is typically targeted to 25% 35% of the total DCI (For a DCI of 2300 kcal) Lower end (25% of DCI): 0.25 2300 kcal = 575 kcal/d Higher end (35% of DCI): 0.35 2300 kcal = 805 kcal/d *Dietitian calculators are available to assist in determining triheptanoin dosage based on DCI, simplifying the calculation process Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023.
Answer: Clinical Case 1 (continued) Based on her DCI, the recommended maintenance dose of triheptanoin for this patient is 69.3 to 97 mL/d, with an initial TDD of around 35 mL/d due to her prior MCT oil intake* 4. Convert to kcals to mL (triheptanoin provides approximately 8.3 kcal/mL) Lower end: (575 kcal) / (8.3 kcal/mL) 69.3 mL/d Higher end: (805 kcal) / (8.3 kcal/mL) 97 mL/d 5. Gradual dose adjustment Add 3 4 mL/d (about 10% increments) to the initial 35 mL/d dose, gradually increasing the dosage to reach the target range of 69 97 mL/d, based on the patient s clinical tolerance and response *Dietitian calculators are available to assist in determining triheptanoin dosage based on DCI, simplifying the calculation process Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023.
Clinical Case 2 A 35-yo male, diagnosed with late-onset carnitine palmitoyltransferase II deficiency at age 22 y after recurrent episodes of rhabdomyolysis, presents with persistent abdominal pain and diarrhea. He was recently started on 70 mL triheptanoin. Physical examination shows no significant findings. He weighs 70 kg. Laboratory tests reveal normal blood glucose and creatine kinase levels, but mild electrolyte imbalances consistent with diarrhea are noted. At this time, what is the most appropriate next step(s) in management?
Answer: Clinical Case 2 The patient's symptoms suggest intolerance to the current triheptanoin dose. The best next step(s) in management include: Monitor electrolytes: Address any electrolyte imbalances resulting from ongoing diarrhea to prevent further metabolic issues. Correct administration: Ensure triheptanoin is properly administered, such as mixing it with food, to reduce gastrointestinal discomfort. Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023.
Answer: Clinical Case 2 (continued) The patient's symptoms suggest intolerance to the current triheptanoin dose. Dosage adjustment: Consider reducing the dose or dividing it into smaller, more frequent doses to help minimize side effects while maintaining therapeutic efficacy. If needed, lower the dose to the last tolerated level and restart with a slower titration. If still not tolerated, stop at the highest tolerated dose for maximum benefit. Ultragenyx Pharmaceutical Inc. Dojolvi (triheptanoin) prescribing information. Novato, CA; October 2023.
Point-of-Care Resource for Healthcare Teams That Provide Care to Patients With Long-Chain Fatty Acid Oxidation Disorders (LC-FAODs) I) Understanding LC-FAODs II) Triheptanoin Therapy Definition and characteristics Impact on patient quality of life Role in LC-FAOD treatment Indications Recommended dosage and administration Monitoring and safety considerations IV) Additional Resources and Support III) Patient Education and Counseling Patient advocacy groups Educational materials and online communities Lifestyle modifications and dietary considerations Symptoms and complications of LC-FAODs