Functional Groups in Organic Compounds
Explore different functional groups in organic compounds, including amines, amides, nitriles, alkyl halides, alkenes, alkynes, and aromatic rings. Learn about their structures, properties, and significance in organic chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
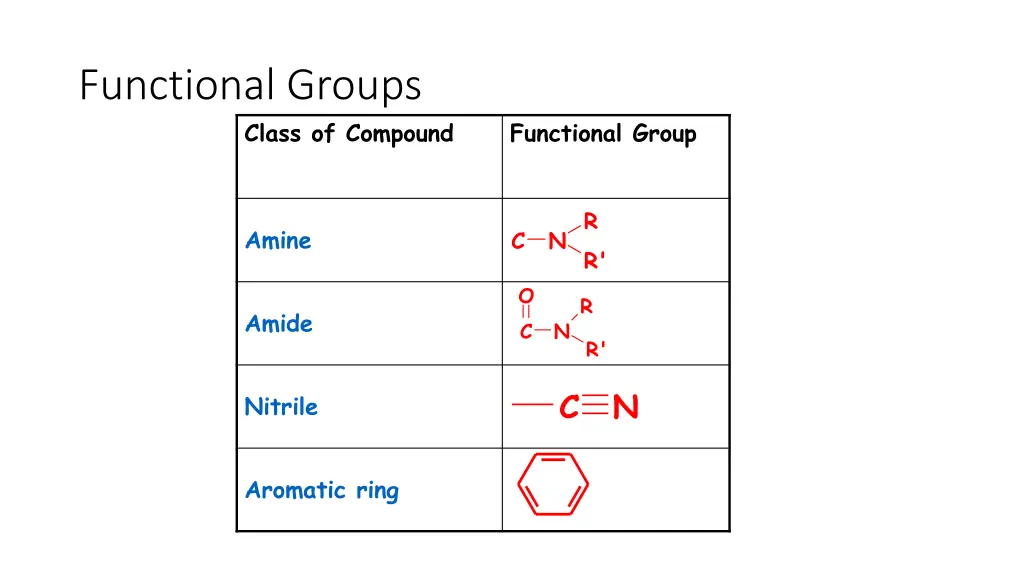
Functional Groups Class of Compound Functional Group R R' Amine C N O R Amide C N R' C N Nitrile Aromatic ring
Alkanes H H H Contain C-C single bonds no functional group Tetrahedral electron domain geometry H HC H H H H C C H H H H C C C C H H H C H H H H H H CH3CH2CH3 C CC H H C HH H C C CH3CH2CH3 CH3CH2CH3 H C H H H H H H H H H sp3hybridized carbons C C H H H C C C H H H H H H H Free rotation around single bonds H H H propane H C C CH3CH2CH3 H H C C H H H H H H H C C H H H H
Cycloalkanes Contain C C with at least 3 of the carbons arranged in a cyclic (ring) structure No functional group H H H H H c c c H H c H c H H Tetrahedral sp3 hybrid orbitals CH2CH2CHCH2CH3
H C C CH3 C C H H H C C H H C C CH3 C C H H C C CH3 H C C C H C C H C H H Alkyl Halides H H CH2=CHCH3 H H H C C H H C C H H H C H H C H C H H H C H H C H C C H C Contain C-halogen bond F, Cl, Br, or I CH2=CHCH3 HH H HH C H C H C Br H C H CH2=CHCH3 H H H H H H H H C H C H H C Br H C H C H C H H C Br H C H CH3CH(Br)CH2CH3 Br CH3CH(Br)CH2CH3 Br CH3CH(Br)CH2CH3 Br
Alkenes H H C C Contain C=C (carbon-carbon double bonds) 1 sigma bond & 1 pi bond H H H CH3 H H H C C C C H C CH3 H Trigonal planar geometry H H CH2=CHCH3 CH2=CHCH3 Which atoms must be coplanar in an alkene? H H H C C H H C H 1-propene sp2 hybridized carbons CH2=CHCH3
Alkenes The C=C present in an alkene is composed of 1 sigma ( ) bond and 1 pi ( ) bond. C C H H H H ethylene Double bonds are rigid and cannot rotate freely. Rotation would cause loss of overlap of the p orbitals, destroying the bond.
H C C CH3 C C Alkynes C H H H C C CH3 C H H C C Contain C C triple bonds 1 sigma bond 2 pi bonds Linear electron domain geometry H H H H H C H C C H C H H H C C CH3 C C CH2=CHCH3 H H C C CH3 C C C C H H C H C C H C HH CH2=CHCH3 H H H Which atoms must be co-linear in an alkyne? H H C C C sp hybridized carbons H HH H C C H C H H CH2=CHCH3 CH2=CHCH3
Ph H C H H CH3CHCH3 H C C Aromatic Ring Planar ring system with alternating single and double bonds does not react like an alkene H C H H C C C C C C C C H H H C C C C C H C H C H H benzene C H C H C H H C Ph C H CH3CHCH3 Trigonal planar C H H C C C C CH3CHCH3 C H N H CH3CHCH3 Ph C C C sp2 hybridized carbons .. C C H Ph C C C H H Benzene ring is a very common aromatic ring. C C Ph N C H N H pyridine C C C .. H C C H C H H C C C C C H H C C C C C H C H H C C C C H C H H C C C C C C C
Functional Groups Alkanes are often called saturated hydrocarbons Organic compounds composed of carbon and hydrogen that contain the largest possible number of hydrogen atoms per carbon atom. Alkenes, alkynes, and aromatic hydrocarbons are called unsaturated hydrocarbons Organic compounds composed of carbon and hydrogen that contain less hydrogen than an alkane having the same number of carbon atoms
