GADOLIN: A Phase III Trial of Obinutuzumab and Bendamustine
These Phase III trials evaluate the efficacy of Obinutuzumab in combination with other agents for different types of lymphoma, showing promising results in terms of progression-free survival (PFS) and overall survival (OS). The studies compare Obinutuzumab with Bendamustine, Rituximab, and CHOP regimens, highlighting the benefits and outcomes observed in each trial. Adverse events and select outcomes from GALLIUM trial are also presented. Additionally, the comparison between PI3 Kinase Inhibitors Idelalisib and Copanlisib is discussed, showcasing the potential advantages of broader-targeting PI3 Kinase inhibitors. The evolving landscape of treatment options in mantle cell lymphoma emphasizes the complexity of therapy sequencing.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
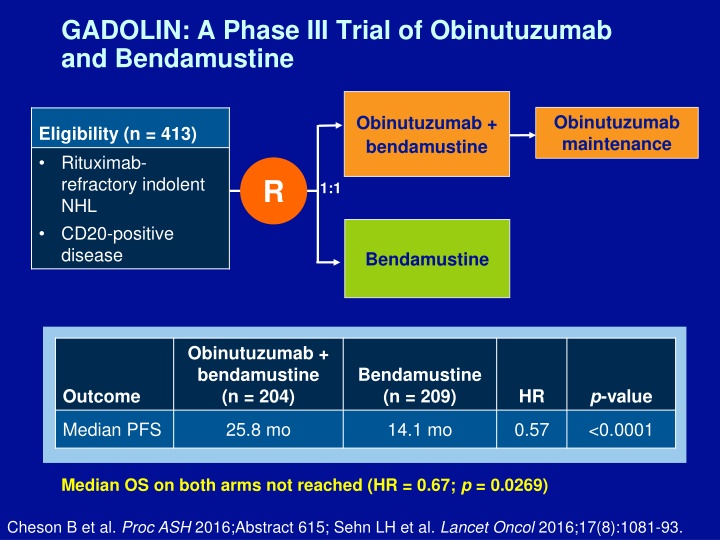
GADOLIN: A Phase III Trial of Obinutuzumab and Bendamustine Obinutuzumab maintenance Obinutuzumab + bendamustine Eligibility (n = 413) Rituximab- refractory indolent NHL CD20-positive disease R 1:1 Bendamustine Obinutuzumab + bendamustine (n = 204) Bendamustine (n = 209) p-value Outcome HR Median PFS 25.8 mo 14.1 mo 0.57 <0.0001 Median OS on both arms not reached (HR = 0.67; p = 0.0269) Cheson B et al. Proc ASH 2016;Abstract 615; Sehn LH et al. Lancet Oncol 2016;17(8):1081-93.
GALLIUM: A Phase III Trial of Obinutuzumab- Based Induction and Maintenance Therapy Target accrual (n = 1,401) Obinutuzumab + chemotherapy Obinutuzumab maintenance Eligibility Previously untreated indolent NHL CD20-positive disease R 1:1 Rituximab + chemotherapy Rituximab maintenance Obinutuzumab + chemotherapy (n = 601) Rituximab + chemotherapy (n = 601) p-value Untreated FL HR Three-year PFS 80.0% 73.3% 92.1% 0.66 0.0012 Three-year OS 94.0% 0.75 0.21 Marcus RE et al. Proc ASH 2016;Abstract 6; www.clinicaltrials.gov. NCT01332968.
GOYA: A Phase III Trial of Obinutuzumab/CHOP or Rituximab/CHOP for Untreated DLBCL Obinutuzumab + CHOP Eligibility (n = 1,418) Previously untreated DLBCL CD20-positive disease R 1:1 Rituximab + CHOP Obinutuzumab + CHOP (n = 706) Rituximab + CHOP (n = 712) p-value Outcome HR Three-year PFS 69% 66% 0.92 0.3868 Three-year OS 81% 81% 0.99 0.9890 Vitolo U et al. Proc ASH 2016;Abstract 470; www.clinicaltrials.gov. NCT01287741.
GALLIUM: Select Adverse Events Obinutuzumab + chemotherapy (n = 597) Rituximab + chemotherapy (n = 595) Grade 3 adverse event Neutropenia 43.9% 37.9% Infusion-related reaction occurring within 24 hours of infusion 12.4% 6.7% Febrile neutropenia 6.9% 4.9% Marcus RE et al. Proc ASH 2016;Abstract 6.
PI3 Kinase Inhibitors: Idelalisib versus Copanlisib Idelalisib First-in-class PI3 kinase inhibitor Targets the PI3 kinase delta isoform Copanlisib PI3 kinase inhibitor currently under development Known to target PI3 kinase alpha and delta isoforms. It's thought that by targeting a broader scope of PI3 kinase it might have an advantage Laurie H Sehn, MD, MPH
Approach to Sequencing Therapies in MCL The world s getting more complicated for mantle cell lymphoma, because we are starting to have more and more options become available There s no right or wrong sequence in a patient with mantle cell lymphoma. As all patients, you need to look at their clinical condition; not only what their lymphoma is doing, but who the patient is. What are the treatment options you have available? What are the toxicities of that treatment? And try and match it up with the patient. So there s no standard sequence in my mind. All of these options will likely be used, because we know that we still don t have a curative strategy for patients with mantle cell lymphoma. Laurie H Sehn, MD, MPH
AETHERA: A Phase III Trial of Brentuximab Vedotin for Hodgkin Lymphoma (HL) Brentuximab vedotin 1.8 mg/kg q3wk (n = 165) Eligibility (n = 329) Patients at high risk of residual HL after autologous stem cell transplant (ASCT) R 1:1 Placebo (n = 164) Moskowitz CH et al. Lancet 2015;385(9980):1853-62.
AETHERA: A Phase III Trial of Brentuximab Vedotin for HL Brentuximab vedotin 1.8 mg/kg q3wk Eligibility (n = 329) Patients at high risk of residual HL after ASCT R 1:1 Placebo Brentuximab (n = 165) 42.9 mo Placebo (n = 164) 24.1 mo p-value 0.0013 Outcome Median PFS HR 0.57 After a median observation time of 30 months, interim analysis of OS showed no significant difference between treatment groups Moskowitz CH et al. Lancet 2015;385(9980):1853-62.
Case Discussion A 72-year-old woman presented in December 2013 with widespread diffuse lymphadenopathy, fatigue and night sweats. Fine needle aspirate biopsies and core biopsies were inconclusive. Cervical lymph node biopsy showed PTCL-NOS. Staging revealed Stage IV disease. Patient received CHOP x 3 cycles without any benefit. Treatment was switched to a gemcitabine-based regimen and achieved a near PR with symptomatic improvement
Case Discussion A 72-year-old woman presented in December 2013 with widespread diffuse lymphadenopathy, fatigue and night sweats. Fine needle aspirate biopsies and core biopsies were inconclusive. Cervical lymph node biopsy showed PTCL-NOS. Staging revealed Stage IV disease. Patient received CHOP x 3 cycles without any benefit, then gemcitabine-based chemotherapy. Upon disease progression, patient received romidepsin x 6 cycles
First-Line Therapeutic Approach for CLL We are going to see a lot of changes in the management algorithm, where people probably try to trend away from the high-intensity, high-toxicity up-front therapy. And these novel targeted agents undoubtedly are going to be making their way more typically into the front-line setting... In my own clinic, we have funding for ibrutinib up front for patients with 17p deletion, where we know that chemotherapy is not very effective For an elderly patient who is not a candidate for chemotherapy and rituximab, I do think about using chlorambucil and obinutuzumab, which, again, has been shown to be a very effective combination for patients who are elderly and who you don t want to treat with a more toxic regimen. Laurie H Sehn, MD, MPH
Ibrutinib for CLL and SLL: Long-Term Safety Analysis After 5 Years of Follow-Up N = 330 Grade 1-2 Grade 3-4 Grade 5 Diarrhea 48% 5% 0 Arthralgia 21% 2% 0 Hypertension 13% 7% 0 Rash 33% 4% 0 Bleeding 50% 5% <1% Atrial fibrillation 7% 5% 0 Coutre S et al. Proc ASH 2016;Abstract 4383.
Management of Tumor Lysis Associated with Venetoclax We know for patients with CLL and possibly higher tumor burden, high-grade lymphomas that tumor lysis syndrome is a definite risk with that drug. As it rolls out into the clinic and it s starting to be used more commonly now, I think that individual doctors are going to need to have a strategy for assessing the risk of tumor lysis syndrome in individual patients, and then managing that patient accordingly, particularly in their first cycle. So the good thing is that this is generally a risk only within the initiation of treatment, and that risk goes away shortly thereafter. But with the initial dosing of the drug, there does need to be a strategy in place of identifying higher-risk patients and then monitoring for the risk of tumor lysis syndrome. Laurie H Sehn, MD, MPH
Consolidation and Maintenance Therapy with RVD for Patients with High-Risk MM Identifying patients with high-risk MM at the time of diagnosis is important: If that opportunity is missed, the high risk will be difficult to determine until the patient has experienced notably early relapse. It is important to use aggressive treatment, both as up-front induction and as maintenance therapy: If this is done, the long-term outcomes can ultimately be improved. Sagar Lonial, MD Lonial S et al. Blood 2015;126:1536-43.
IFM/DFCI 2009: A Phase III Trial of ASCT for MM Lenalidomide maintenance x 1 y RVd Eligibility (n = 700) Previously untreated MM R 1:1 Lenalidomide maintenance x 1 y RVd + ASCT RVd RVd + ASCT (n = 350) 58% p-value <0.01 Outcome CR rate (n = 350) 46% HR Attal M et al. Proc ASH 2015; Abstract 391.
IFM/DFCI 2009: A Phase III Trial of ASCT for MM RVd Lenalidomide maintenance x 1 y (n = 350) Eligibility (n = 700) Previously untreated MM R 1:1 Lenalidomide maintenance x 1 y RVd + ASCT (n = 350) Three-year PFS for patients achieving CR MRD negativity by NGS (<10-6) MRD positivity by NGS ( 10-6) Before maintenance therapy 87% 63% After maintenance therapy 92% 64% Avet Loiseau H et al. Proc ASH 2015;Abstract 191.
Personal Experience with Daratumumab in Combination with an IMiD or Immune-Directed Therapy The effects of an IMiD on a myeloma cell are to ultimately kill that myeloma cell, but the effect of that same drug on a T cell or an NK cell is to actually activate that immune cell. What really strikes that home to me is, we ve treated a number of patients that are either resistant to pom or dara or both with both drugs together, and we ve seen at least 30% of patients respond. Sagar Lonial, MD
Case Discussion A 75-year-old woman diagnosed with indolent MM Received RVd, underwent ASCT and received maintenance single-agent lenalidomide for about 2 months before suddenly deciding to stop maintenance therapy Received no maintenance therapy for about 2 years Patient experienced a biochemical relapse
Case Discussion A 75-year-old woman diagnosed with indolent MM Received RVd, underwent ASCT and received maintenance single-agent lenalidomide for about 2 months before suddenly deciding to stop maintenance therapy Received no maintenance therapy for about 2 years before experiencing a biochemical relapse Patient is currently receiving ixazomib/lenalidomide/dexamethasone
Toxicities Associated with Ixazomib Therapy In the up-front setting, we ve seen some GI toxicity that actually is mitigated by the dex, which seems to help a little bit. In the maintenance setting, when we ve tried to use it there at full dose, we ve had a little bit of trouble giving 4 mg in the very beginning If you start with 3 for a couple of cycles and then work your way up, you can get to 4 without significant issues. Most of the skin rash and nausea that was seen was seen at the higher doses of ixazomib. At the dose that was settled on now, the fixed dose, it s a much lower incidence. Sagar Lonial, MD
Phase I Study of Venetoclax Monotherapy for Relapsed/Refractory MM Without t(11;14) or undetermined (n = 36) All patients (n = 66) With t(11;14) (n = 30) Response ORR 21% 40% 6% Kumar S et al. Proc ASH 2016;Abstract 488.
Utility of Chimeric Antigen Receptor T (CAR T)- Cell Therapy versus BiTEs in MM In my view, CAR T cells are important. They re clearly effective, and they will be a way that we treat many diseases down the road. We are also beginning to develop BiTEs in myeloma, the bispecific antibodies, so CD3 partnered with BCMA or CD3 partnered with CD38. These, I think, will offer similar efficacy to what we see with the CAR T cells. The advantage of the BiTE, in my view, will be that it s an off-the-shelf preparation. You don t have to go through all this process of making a cell, finding problems with the manufacturing process. With the BiTE, you pull it off the shelf and you give it. Sagar Lonial, MD
Case Discussion A 54-year-old woman with relapsed/refractory AML Received MEK inhibitor-based chemotherapy and experienced a short-lived second remission What are the therapeutic options for this patient?
Case Discussion A 54-year-old woman with relapsed/refractory AML Received MEK inhibitor-based chemotherapy and experienced a short-lived second remission Bone marrow biopsy has been ordered and FLT3 mutation testing is being performed
Case Discussion A 54-year-old woman with relapsed/refractory AML Received MEK inhibitor-based chemotherapy and experienced a short-lived second remission Bone marrow biopsy has been ordered FLT3 mutation testing is being performed If testing reveals FLT3 mutation-positive disease, which FLT3 inhibitor would you recommend?
Phase III CALGB-10603 (RATIFY) Trial of Midostaurin for Newly Diagnosed FLT3 Mutation- Positive AML1 Midostaurin (n = 360) Placebo (n = 357) p-value Outcome HR Median OS 74.7 mo 26.0 mo 0.77 0.007 Phase I/II Trial of Gilteritinib for Relapsed/ Refractory AML2 FLT3 mutation-positive 20-450 mg (n = 127) FLT3 wild type 20-450 mg (n = 57) 80 mg (n = 106) Outcome ORR 52% 57.5% 8.8% 1 Stone RM et al. Proc ASH 2015;Abstract 6; 2 Levis MJ et al. Proc ASCO 2015;Abstract 7003.
Approach to FLT3 Mutation Testing in AML After the diagnosis of AML: Cytogenetic or AML-specific FISH analysis should be performed. Karyotype testing should be performed. NPM1 and FLT3 mutation status should be determined up front. Raoul Tibes, MD, PhD
Phase Ib/II Study of Venetoclax and Low-Dose Cytarabine for Untreated AML in Patients 65 Years and Older 600 mg venetocax (n = 16) 800 mg venetoclax (n = 10) All patients (n = 26) Response ORR 12 (75%) 3 (30%) 15 (58%) CR + CRi 11 (69%) 3 (30%) 14 (54%) All patients (n = 26) Patients without MPN (n = 22) Survival One-year OS 57.6% 70.5% Lin TL et al. Proc ASCO 2016;Abstract 7007.
Case Discussion A 36-year-old man with newly diagnosed chronic- phase CML Normal renal function Normal platelet count, hemoglobin 11.8 g/dL Received hydroxyurea/allopurinol Peripheral blood FISH revealed t(9;22) BCR-ABL. No blasts
Case Discussion A 36-year-old man with newly diagnosed chronic- phase CML Normal renal function Normal platelet count, hemoglobin 11.8 g/dL Received hydroxyurea/allopurinol Peripheral blood FISH revealed t(9;22) BCR-ABL. No blasts Which BCR-ABL TKI would you recommend?
Case Discussion A 36-year-old man with newly diagnosed chronic- phase CML Normal renal function Normal platelet count, hemoglobin 11.8 g/dL Received hydroxyurea/allopurinol Peripheral blood FISH revealed t(9;22) BCR-ABL. No blasts Patient received nilotinib
Decision-Making Approach to the Use of JAK Inhibitors for Patients with MPN The way I use JAK inhibitors is for intermediate and high-risk patients, if they are symptomatic according to the DIPSS or DIPSS Plus Prognostic Scoring System Does the patient have cachexia, or does he have splenomegaly? Does he have weight loss, night sweats, and so forth? JAK2 inhibitors are very good at controlling symptoms in MPNs. They do also control the leukocytosis, as well as erythrocytosis. So increased counts they can control as well. So if a patient has an intermediate or higher-risk myelofibrosis, is symptomatic, I would start a patient on a JAK2 inhibitor. Raoul Tibes, MD, PhD
Case Discussion A 36-year-old woman with newly diagnosed Ph- negative ALL Received a pediatrics-modeled induction regimen for young adults after the CALGB-10403 trial Developed disease progression after 2 cycles Patient received blinatumomab allogeneic SCT
Achieving MRD-Negative Status with Blinatumomab in the Management of ALL We now have a drug, blinatumomab, that actually can achieve remission in patients with refractory ALL, in a very high percentage, as well as good data now, emerging data, that blinatumomab can convert MRD- positive disease to MRD-negative disease MRD, or minimal residual disease, monitoring is very important in ALL. And I encourage all of my colleagues to send it out, so we do not just fall in morphological remission, but we also want to see MRD remissions. That s similar to CML, where we measure BCR-ABL. We have various ways of measuring, by flow or by PCR, MRD for ALL. Raoul Tibes, MD, PhD
Case Discussion A 62-year-old man with a history of Stage III non- GCB DLBCL Received R-CHOP x 6 cycles and consolidation radiation therapy for bulky disease Developed primary refractory disease to multiple therapies
Case Discussion A 62-year-old man with a history of Stage III non- GCB DLBCL Received R-CHOP x 6 cycles and consolidation radiation therapy for bulky disease Developed primary refractory disease to multiple therapies Patient received lenalidomide/rituximab for 4 months and experienced stable disease
Case Discussion A 62-year-old man with a history of Stage III non- GCB DLBCL Received R-CHOP x 6 cycles and consolidation radiation therapy for bulky disease Developed primary refractory disease to multiple therapies Received lenalidomide/rituximab for 4 months and experienced stable disease Patient received CAR T-cell therapy on a clinical trial and experienced a durable complete response
Is Sequencing CAR T-Cell Therapy After Lenalidomide/Rituximab Beneficial? We generally do like to see some sense of response before I will take patients to CAR T. And what I mean by that, if I can debulk them some, I generally think that they will have a better outcome. Now, this has not been described in prospective studies, but we do know that the CAR Ts are currently associated with pretty high toxicity. And so if we can have some idea that they re going to respond to an immune-therapy approach with something such as lenalidomide, and if I can reduce the proliferative rate prior to proceeding with CAR T, I m much more optimistic about the outcomes in those patients. Loretta J Nastoupil, MD
Overview of CAR T-Cell Therapy A E Leukapheresis Modified T-cell infusion Antibody- coated beads D B Chemotherapy T-cell activation/transduction Bead removal C Modified T-cell expansion
Case Discussion A 62-year-old man with a history of Stage III non- GCB DLBCL Received R-CHOP x 6 cycles and consolidation radiation therapy for bulky disease Developed primary refractory disease to multiple therapies; received lenalidomide/rituximab for 4 months and experienced stable disease Received CAR T-cell therapy on a clinical trial and experienced a durable complete response Patient experienced fever and difficulty with word finding and orientation while receiving CAR T-cell therapy
Management of Toxicities Associated with CAR T-Cell Therapy We have picked up some subclinical seizure activity, and we ve had patients that have had status epilepticus. So it s a big spectrum there... The cytokine release syndrome, though not to minimize that concern, generally speaking, I think we re successful at identifying patients that become hemodynamically unstable. We can manage them appropriately. I think the neurotoxicity is a little bit more challenging, in my opinion, because it s more unpredictable and it s a little bit harder to tease out what is the cause We don t know if it s the CAR Ts that are compromising the blood-brain barrier and causing cytokine release within the brain or if it s something else. Loretta J Nastoupil, MD
ZUMA-1: A Phase II Trial of CAR T-Cell Therapy for Refractory DLBCL Response (N = 51) ORR CR PR All patients 76% 47% 29% Chemotherapy refractory 76% NR NR Post-ASCT relapse 80% NR NR Responses at 1 month 92% NR NR Ongoing responses at 3 months 39% 33% 6% NR = not reported Neelapu SS et al. Proc ASH 2016;Abstract LBA-6.
ZUMA-1: CAR T-Cell Therapy Side Effects N = 101 Grade 3 Neutropenia 67% Anemia 39% Neurologic events 29% Febrile neutropenia 27% Cytokine release syndrome 20% Neelapu SS et al. Proc ASH 2016;Abstract LBA-6.
ACE-CL-001: A Phase I/II Trial of Acalabrutinib for Relapsed CLL Response ORR All evaluable patients (n = 60) 95% Del(17p13.1) (n = 18) 100% Byrd JC et al. N Engl J Med 2016;374(4):323-32.
ACE-CL-001: A Phase I/II Trial of Acalabrutinib for Relapsed CLL Response ORR All evaluable patients (n = 60) 95% Del(17p13.1) (n = 18) 100% No cases of major bleeding or atrial fibrillation at 14.3 months of follow-up Byrd JC et al. N Engl J Med 2016;374(4):323-32.
Phase III RELEVANCE Trial Target accrual (n = 1,031) Lenalidomide/rituximab (R2) Eligibility CD20-positive FL (Grade I, II or IIIA) R Stage II-IV disease Rituximab/chemotherapy* No prior systemic therapy *R-CHOP, R-CVP or bendamustine/rituximab Primary endpoints: PFS and complete response rate www.clinicaltrials.gov. NCT01650701 (Accessed March 2017).
Experience with Toxicities Associated with the R2 Regimen The side-effect intensity seems to be higher in the first few months and then tends to stabilize or improve over time: The first 2 months with R2 are not a free lunch. We have seen fever, rashes, myalgias and neutropenia: Fortunately, despite the Grade 3 and 4 neutropenias, we ve not seen much in the way of infections. R2 has a different safety profile from what we generally see with chemotherapy-based approaches, and it has unique side effects that warrant discussing with patients. Loretta J Nastoupil, MD