Gastrointestinal Agents and Gastric Gland Cells
Role of gastrointestinal agents in managing disorders like altering gastric pH, constipation, and intestinal inflammation. Learn about the stomach's secretion, gastric gland cells, and the production of hydrochloric acid by parietal cells. Discover how hydrochloric acid kills bacteria, softens food, and aids in digestion.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Gastrointestinal Agents Dr. Ghufran Mohammed Hussein
Gastrointestinal Agents Any inorganic substances which can be used in gastrointestinal disorder are called gastrointestinal agent
Classification of inorganic gastrointestinal agents Products for altering gastric pH: 1. a) Acidifying agents (induce acid secretion, e.g. histamine phosphate) b) Gastric Antacids, e.g. Al(OH)3 and NaHCO3 Protectives for intestinal inflammation, e.g. bismuth 2. subcarbonate. Adsorbents for intestinal toxins, e.g. activated charcoal 3. Cathartics or laxatives for constipation 4. Antiemetic agents 5.
The stomach secretes: Hydrochloric acid (HCl) Bicarbonate Pepsinogen Intrinsic factor Mucus Prostaglandins
Cells of the Gastric Gland Parietal cells Produce and secrete HCl Primary site of action for many acid-controller drugs Chief cells Secrete pepsinogen (proenzyme) Pepsinogen becomes pepsin when activated by exposure to acid Pepsin breaks down proteins (proteolytic) Mucoid cells Mucus-secreting cells (surface epithelial cells) Provide a protective mucous coat Protect against self-digestion by HCl
Hydrochloric Acid Secreted by the parietal cells when stimulated by food Maintains stomach at pH of 1 to 4 Secretion also stimulated by: Large fatty meals Excessive amounts of alcohol Emotional stress
Kills the bacteria in ingested food and drink Softens fibrous foods Promotes the formation of pepsin that helps in digestion Lack of HCl can cause gastrointestinal disturbances.
HCl Production Parietal cells secrete H+ into gastric lumen by primary active transport, through H+/ K+ ATPase pump. Parietal cell s basolateral membrane takes in Cl- against its electrochemical gradient, by coupling its transport with HC03-.
A. Achlorhydria Is the medical term for a lack of stomach acid (HCl) due to failure of the parietal cells to produce gastric acid. Patients with this condition fall into one of the following two groups: 1. Patients remain free of gastric HCl after stimulation with histamine phosphate. Treatment: Diluted HCl has been utilized. 2. Patients in whom there is normal lack of gastric HCl but will secrete it upon stimulation by histamine. Treatment: Administration of histamine phosphate.
B. Gastric hyper secretion: Hyperacidity: an excess amount of gastric HCl is secreted. When hyperacidity develops, the results can range from gastritis (a general inflammation of gastric mucosa) to peptic ulcer (a specific erosion).
Peptic Ulcer: Break in continuity of mucosa of any part of GIT caused by HCl and pepsin. Peptic ulcer may be located: Lower end of the esophagus (esophageal ulcer) Stomach (gastric ulcer) Duodenum (duodenal ulcer)
An ulcer occurs when stomach acid eats away the inner lining of the digestive tract. Normally, there is a layer of mucus to protect the gastrointestinal lining from the harsh acid. But if there is excess acid or inadequate mucus, then the chances of developing an ulcer increases. Most experts agree that ulcers are either caused by bacteria or medications.
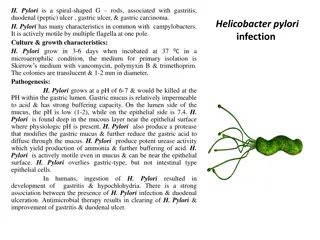
Helicobacter pylorus (H. pylori) is one of the most common causes of peptic ulcers. H. pylori is a bacteria that lives within the protective mucus lining the stomach or small intestine, but it can also disrupt mucosal activity and lead to an ulcer. NSAIDs such as aspirin can cause ulcers when used on a regular basis, as when used to prevent heart attack and stroke.
Depends on the cause. Usually treatment will involve killing the H. pylori bacterium, if present, eliminating or reducing use of NSAIDs, and helping your ulcer to heal with medication. Antibiotic medications to kill H. pylori. Medications that block acid production and promote healing. Medications to reduce acid production. Antacids that neutralize stomach acid. Medications that protect the lining of your stomach and small intestine.
Heart Burn Heartburn: is a painful burning feeling in your chest or throat. It happens when stomach acid backs up into your esophagus, the tube that carries food from your mouth to your stomach. Causes: Overeating Alcohol consumption Eating certain foods Anxiety Smoking Certain Drugs, i.e. Aspirin
Heart Burn Treatment Apart from lifestyle alterations, heartburn can be reduced by using drugs such as: Antacids 1. Proton-pump inhibitors (PPIs) 2. Histamine-2 blockers 3.
Antacids Antacids are substances which reduce gastric acidity resulting in an increase in the pH of stomach and duodenum. It is itself basic in nature. Weak bases are used for this purpose. e.g. NaHCO3, Al(OH)3, Mg(OH)2 and CaCO3
Antacids Antacids are drugs that are widely used to: Relief uncomfortable feeling from overeating Heartburn Dyspepsia (indigestion) Other non-specific GI symptoms
Criteria of an Ideal Antacid The antacid should not be absorbable or cause systemic 1. alkalosis The antacid should not be laxative or cause constipation 2. The antacid should exert its effect rapidly and over a long 3. period of time The antacid should buffer in the pH 4-6 range 4. The reaction of the antacid with gastric hydrochloric acid 5. should not cause a large evolution of gas
Action of Antacids Sodium bicarbonate NaHCO3 (aq) + HCl (aq) NaCl (aq) + H2O (l) + CO2 (g) Magnesium hydroxide Mg(OH)2 (aq) + 2 HCl (aq) MgCl2 (aq) +2 H2O (l) Aluminium hydroxide Al(OH)3 (s) + 3 HCl (aq) AlCl3 (aq) + 3 H2O (l) Calcium carbonate CaCO3 (s) + 2HCl (aq) CaCl2 (aq) + H2O (l) + CO2 (g)
Side Effects of Antacid Therapy 1. Acid rebound: If the gastric pH is raised too much acid rebound may occur since in an effort to maintain a lower pH, the stomach secretes additional hydrochloric acid which consumes the antacid. The result is that an excess acid is secreted into the stomach, leading to a hyper acidic condition, which could further aggravate the ulcer.
Side Effects of Antacid Therapy 2. Systemic alkalosis: If the antacid is sufficiently water soluble and is composed of readily absorbable ions, the antacids may be absorbed and exert its alkaline effects on body s buffer systems. 3. Constipating effects: Antacids containing calcium and aluminum ions tend to have a constipating effects. 4. Laxative effects: Antacids containing magnesium salt tend to have a laxative effects