Grazoprevir and Elbasvir Combination Study in Genotype 3 HCV Patients
Explore the results of the C-WORTHY Study Part D, which focused on the efficacy of grazoprevir and elbasvir combination therapy with ribavirin in treating genotype 3 hepatitis C virus (HCV) patients. The study included baseline characteristics, patient disposition, SVR12 rates, resistance-associated substitutions in virologic failure cases, and more. Findings indicate promising SVR12 rates, along with specific NS3 and NS5A resistance substitutions observed in patients experiencing treatment failure.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
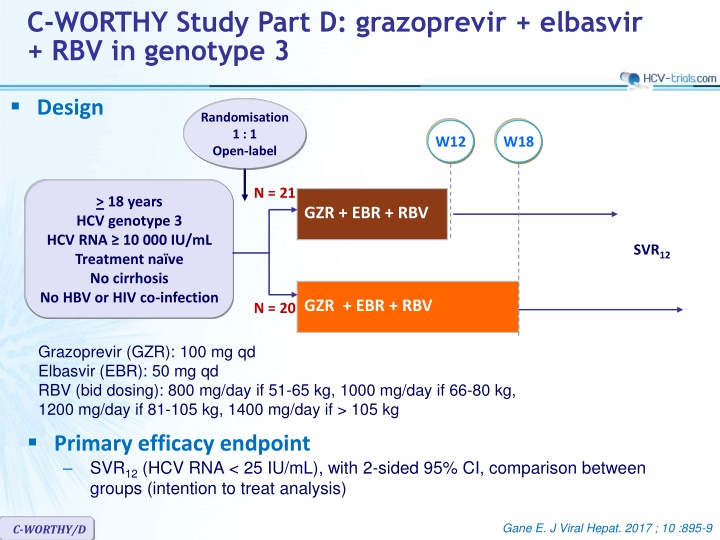
C-WORTHY Study Part D: grazoprevir + elbasvir + RBV in genotype 3 Design Randomisation 1 : 1 Open-label W12 W18 N = 21 > 18 years HCV genotype 3 HCV RNA 10 000 IU/mL Treatment na ve No cirrhosis No HBV or HIV co-infection GZR + EBR + RBV SVR12 GZR + EBR + RBV N = 20 Grazoprevir (GZR): 100 mg qd Elbasvir (EBR): 50 mg qd RBV (bid dosing): 800 mg/day if 51-65 kg, 1000 mg/day if 66-80 kg, 1200 mg/day if 81-105 kg, 1400 mg/day if > 105 kg Primary efficacy endpoint SVR12(HCV RNA < 25 IU/mL), with 2-sided 95% CI, comparison between groups (intention to treat analysis) Gane E. J Viral Hepat. 2017 ; 10 :895-9 C-WORTHY/D
C-WORTHY Study Part D: grazoprevir + elbasvir + RBV in genotype 3 Baseline characteristics and patient disposition GZR + EBR + RBV 12 weeks (N = 20) GZR + EBR + RBV 18 weeks (N = 21) Female 60% 62% Age, years (mean) 49 42 IL28B CC 40% 38% HCV RNA 10M IU/ml 75% 76.2% Metavir F0-F2 / F3 95% / 5% 95.2% / 4.8 % Discontinuation On-treatment failure Adverse event Lost to follow-up, Withdrew consent 2 1 0 1 2 0 1 1 Gane E. J Viral Hepat. 2017 ; 10 :895-9 C-WORTHY/D
C-WORTHY Study Part D: grazoprevir + elbasvir + RBV in genotype 3 SVR12(HCV RNA < 25 IU/ml), % (95% CI), ITT % 100 75 57.1 (34.0-78.2) 45.0 50 (23.1-68.5) 25 20 21 0 12 weeks 18 weeks Rebound Breakthrough Non response Relapse 3 6 2 5 0 0 1 (quantifiable virus at TW8) 0 Gane E. J Viral Hepat. 2017 ; 10 :895-9 C-WORTHY/D
C-WORTHY Study Part D: grazoprevir + elbasvir + RBV in genotype 3 Resistance associated substitutions in subjects with virologic failure NS3 RAVs NS5A RAVs 12W arm Breakthrough Breakthrough Rebound Breakthrough Breakthrough Rebound Breakthrough Non response Rebound Breakthrough 18W arm Breakthrough Breakthrough Breakthrough Rebound Rebound Breakthrough Breakthrough At baseline V170I V170I V170I V170I V170I V170I V170I, L132I V170I V170I V170I At failure V170I Q80K na V170I, A156G Q80K, V170I V170I, A156G, Q80K Q80R, V170I, L132I Q80K, V170I Y56Y/H, Q168Q/K, V170I V170I, A156G At baseline A30L WT WT WT WT WT WT A30A/E/K/T, Y93H Y93H WT At failure A30S, Y93Y/H, L31L/I A30G, Y93H na Y93H Y93H Y93H Y93H Y93H Y93H Y93H V170I, Q168R V170I V170I V170I V170I V170I V170I V170I, Q168R Q80K, V170I V170I V170I, A156G A156G Q80K, V170I Q80K WT WT WT WT A30K WT WT L31F L31F Y93H Y93H A30K, P58S Y93H Y93H Gane E. J Viral Hepat. 2017 ; 10 :895-9 C-WORTHY/D
C-WORTHY Study Part D: grazoprevir + elbasvir + RBV in genotype 3 SVR12according to baseline RASs Overall Population GZR + EBR + RBV 12 weeks GZR + EBR + RBV 18 weeks Overall efficacy, n/N (%) 21/38 (55%) 9/19 (47%) 12/19 (63%) NS3 RASs not detected, n/N (%) 3/3 2/2 1/1 (100%) (100%) (100%) NS3 RASs at baseline, n /N (%) 18/35 (51%) 7/17 (41%) 11/18 (61%) NS5A RASs not detected, n/N (%) 14/21 (67%) 5/9 (56%) 9/12 (75%) NS5A RASs at baseline, n/N (%) 7/17 (41%) 4/10 (40%) 3/7 (43%) Gane E. J Viral Hepat. 2017 ; 10 :895-9 C-WORTHY/D
C-WORTHY Study Part D: grazoprevir + elbasvir + RBV in genotype 3 Adverse events, N (%) GZR + EBR + RBV 12 weeks (N = 20) GZR + EBR + RBV 18 weeks (N = 21) Adverse events Headache Upper respiratory tract infection Accidental overdose Insomnia Rash Nausea Asthenia 17 (85.0) 5 (25.0) 3 (15.0) 3 (15.0) 3 (15.0) 3 (15.0) 3 (15.0) 2 (10.0) 19 (90.5) 5 (23.8) 5 (23.8) 2 (9.5) 3 (14.3) 2 (9.5) 5 (23.8) 3 (14.3) Serious adverse event 0 0 Serious drug-related adverse event 0 0 Discontinuation due to adverse event 0 1* (4.8) * dyspnea, fatigue and asthenia 2-3 days after starting treatment Gane E. J Viral Hepat. 2017 ; 10 :895-9 C-WORTHY/D
C-WORTHY Study Part D: grazoprevir + elbasvir + RBV in genotype 3 Summary Efficacy of 12 or 18 weeks of GZR + EBR + RBV in HCV genotype 3 infected patients Was suboptimal due to on-treatment virologic failure in 17 of 41 patients No subject relapsed after the end of therapy NS5A RASs found in 16 of 17 failures, Y93H the most common NS5A RAS identified GZR + EBR + RBV was generally safe and well tolerated Adverse events were slightly more common in the 18-week arm compared with the 12-week arm No differences in serious adverse events or laboratory abnormalities Adverse event considered to be severe in intensity reported in only 1 patient (non-drug related depression during follow-up) Gane E. J Viral Hepat. 2017 ; 10 :895-9 C-WORTHY/D