Guiding Principles for Patient Involvement in Drug Development
Explore Chapter 4 of CIOMS XI focusing on core principles and operational considerations for patient involvement in drug development, including ethical considerations, mutual benefit, training, communication, transparency, and more. Also, details on a possible EUPATI survey initiative to gather patient perspectives across stakeholders in the drug lifecycle.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
CIOMS XI: Chapter 4 - Guiding Principles and Considerations for Patient Involvement July 2019 Beverly Harrison, Head, Patient Support, Janssen Pharmaceuticals Charles Garrigan, Patient Support, Janssen Pharmaceuticals OFFICE OF THE CHIEF MEDICAL OFFICER
Agenda 1. Refocusing chapter objectives 2. Current outline 3. Discussion of possible EUPATI survey Confidential. For J&J internal use only. OFFICE OF THE CHIEF MEDICAL OFFICER 2
Objectives for Chapter 4 Original Chapter Objectives New Chapter Objectives 1. Provide core principles for patient involvement Provide core principles for patient involvement, with select key operational considerations (e.g. training, fair market value, conflict of interest, value of measuring) 2. Recommendations for developing and enabling organizations for patient involvement 3. Operational Considerations Refocus to provide information that is applicable across target stakeholders and can be developed with a reasonable amount of effort. Also reducing overlap with ongoing worked by IMI-PARADIGM 4. Value of measuring patient involvement activities, and approaches to do so Confidential. For J&J internal use only. OFFICE OF THE CHIEF MEDICAL OFFICER 3
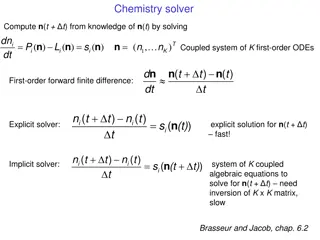
Chapter 4 Outline and Status Section Ethical Considerations for Working with Patients Perspective of Patients and Advocates on Involvement with Drug Development & Safe Use Positioning Patients as Partners Mutual benefit, two-way direction flow of value Training & Education of Stakeholders Training / education of drug developers, regulators, others Training / education of patients Communication & Transparency Importance of ensuring effect and consistent communication Conflict of interest Health literacy, communicating uncertainty Compensation & Fair Market Value Importance of Measuring Applicable Case Studies Status New, being outlined Outlined, considering survey (see next slide) Initial Draft Complete A B C D 50% Drafted E 50% Drafted F G H Outlined Initial Draft Complete Outlined Confidential. For J&J internal use only. OFFICE OF THE CHIEF MEDICAL OFFICER 4
Possible Opportunity for Survey with EUPATI Initial discussion (by Annemiek van Rensen) with EUPATI on doing survey of patient perspective of working with various stakeholders across drug lifecycle EUPATI is open to a formal request for a survey, but ask that we capture all patient survey needs (for entire CIOMS guidance) in one survey Proposed Course of Action: Send out message to entire CIOMS team on intent and to consider specific survey needs, and put on the agenda for next CIOMS in-person meeting in October. Confidential. For J&J internal use only. OFFICE OF THE CHIEF MEDICAL OFFICER 5