HCV and Cirrhosis Patient Case Study: Hispanic Female with Diabetes
This case study details the medical history, medication regimen, and notable labs of a 59-year-old Hispanic female presenting with cirrhosis, diabetes, and other underlying conditions. The patient's background, history of present illness, medication history, patient characteristics, and laboratory results are discussed in this comprehensive analysis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
MM: HCV and Cirrhosis Patient Case Chris Kolkman PrimaryOne Health
Patient Background MM is a 59-year-old Hispanic female who presents for a regular health checkup, accompanied by her daughter to help translate. CC: I would like to establish care here in the US and to have someone manage my diabetes and cirrhosis. Pt reports recently immigrating from Nicaragua, where she had been diagnosed with idiopathic cirrhosis in the past year. Past Medical History (PMH): Carpal Tunnel Dyslipidemia Cirrhosis (Unknown staging, diagnosed May 2022) Type 2 Diabetes Mellitus w/neuropathy (Unknown dx date) Former smoker (Mostly quit 6 months ago)
Cirrhosis History of Present Illness (HPI) Patient reports being diagnosed with cirrhosis in May of 2022, reports no explanation as to the cause. Was prescribed furosemide, spironolactone, and propranolol for this indication, which was restarted in May 2023 during her initial visit. Patient reports that her husband passed away around 12 years ago from cirrhosis, but she likewise received no explanation as to what caused this. Patient denies any illicit injectable drug use.
Medication History Drug Strength Dose & Frequency 1 tab BID Indication Glyburide- Metformin Simvastatin Propranolol Furosemide Spironolactone Gabapentin 2.5-500 mg Diabetes 20 mg 20 mg 20 mg 100 mg 400 mg 1 tab QHS 1 tab BID 1 tab QD 1 tab QD 1 capsule QHS Dyslipidemia Cirrhosis Cirrhosis Cirrhosis Neuropathy *All meds had not been filled for several months but were restarted during initial visit in May.
Patient Characteristics No known drug allergies Social History (SH) BMI 27.74 BP 130/78 Light smoking tobacco use No alcohol use No illicit drug use
Notable Labs Collected 5/5/23 Albumin Alkaline Phosphatase ALT AST Glucose INR A1c HCV Ab HAV Ab LDL Hep C Quantitation Lab Value (Reference) 3.5 g (3.8-4.9 g) 158 IU/L (44-121 IU/L) 45 IU/L (0-32 IU/L) 52 IU/L (0-40 IU/L) 218 mg/dl (70-99 mg/dl) 1.4 (0.9-1.2) 9.1% (4.8-5.6%) Positive (Negative) Positive (Negative) 80 mg/dl(0-99 mg/dl) 357000 IU/mL (0 IU/mL)
Labs Taken at First RPh appointment Collected 5/18 Value (Reference) Hepatitis C Virus Fibrosure 0.50- Stage F2 (0.00- 0.21) HCV Antibody Cascade/PCR Genotyping 2A/2C Genotype (N/A)
Fibrosure Fibrosis Scoring (Metavir) F0 No fibrosis 0.00-0.21 Fibrosure is a blood test that determines fibrosis stage based off 6 markers Alanine aminotransferase (ALT) 2-macroglobulin Apolipoprotein A1 Bilirubin, total -glutamyl transferase (GGT) Haptoglobin Patient s age and sex F0-F1 > 0.21-0.27 F1 Portal fibrosis > 0.27-0.31 F1-F2 > 0.31-0.48 F2 Bridging fibrosis with few septa > 0.48-0.58 F3 Bridging fibrosis with many septa > 0.58-0.72 F3-F4 > 0.72-0.74 F4 Cirrhosis > 0.74-1.00 https://www.labcorp.com/tests/related- documents/L9465#:~:text=%E2%80%A2%20HCV%20FibroSure%20is%20a,necroinflammatory%20activity%2C%20corresponding%20to%20METAVIR
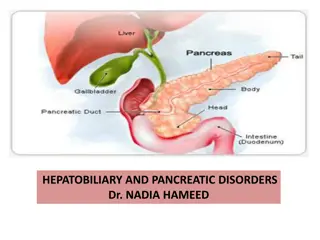
Hepatitis C Virus (HCV)
HCV Approximately half of people who contact HCV will develop a chronic infection and 5-25% of those will develop cirrhosis. HCV is the leading cause of viral hepatitis in the US with 66,700 new cases reported in 2020. Around 40-50% of people with HCV don t know they have it. Virus is transmitted through blood, from mother to baby, and by sexual contact. Almost all cases of HCV are curable with modern medications. Unlike HAV and HBV, there is no vaccine available for HCV. https://www.cdc.gov/hepatitis/abc/index.htm
People at high risk for HCV Infection Baby boomers People who inject illicit drugs People who receive unregulated tattoos or body piercings Babies born to mothers with untreated HCV Men who have sex with men People living with HIV https://www.cdc.gov/hepatitis/hcv/cfaq.htm#transmission
Everyone over 18 should be tested once in their lifetime. Women should be tested during every pregnancy. Who should be screened for HCV? People with abnormal LFTs. People who inject illicit drugs. People living with HIV. Patients on hemodialysis. People who received organ transplants or blood transfusions before 1992. People exposed to HCV+ blood. https://www.cdc.gov/hepatitis/hcv/cfaq.htm#E1
Before Drug Therapy Hep B Surface Antigen testing HIV Antigen/Antibody Testing HCV Viral Load (Tested for with Quantitative HCV RNA) HCV Genotype (If treating with Epclusa) Pregnancy test and counseling about risks of HCV medication to fetus (If patient is a woman of childbearing age) CBC, LFTs, eGFR Potential DDIs with treatment options. The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America
Medication Mechanism of Action Patient Population Dose/Administrat ion Contraindicatio ns Side Effects Epclusa (sofosbuvir/ velpatasvir) HCV NS5B inhibitor + HCV NS5A inhibitor Chronic Hep C patients w/o decompensated cirrhosis. Can be used in decompensated cirrhotic patients if used with ribavirin. Total daily dose: sofosbuvir 400 mg, velpatasvir 100 mg N/A Headache/Fatigue 1 tablet once daily with or without food for 12 weeks Genotypes 1-6 (3 requires resistance testing) Age 6 Mavyret (glecaprevir/ pibrentasvir) NS3/4A protease inhibitor + NS5A inhibitor Chronic Hep C patients w/o decompensated cirrhosis. Total daily dose: glecaprevir 300 mg, pibrentasvir 120 mg Moderate or severe hepatic impairment Headache/Fatigue Genotypes 1-6 History of prior hepatic decompensation 3 tablets once daily with food for 8 weeks Age 3 Weight 99 pounds Epclusa (sofosbuvir and velpatasvir). [package insert]. Mavyret (glecaprevir and pibrentasvir). [package insert].
Literature Supporting Efficacy of Eplcusa in patients with HCV Global real-world evidence of sofosbuvir/velpatasvir as simple, effective hcv treatment: analysis of 5552 patients from 12 cohorts Showed almost 99% of patients obtained sustained virological response at 12 and 24 weeks (SVR12/24) in a diverse patient population. Even patients with compensated cirrhosis had substantial virological response, though slightly lower than those without. Epclusa proved to be effective against all HCV genotypes (1-6). Epclusa also has the benefit of being 1 tablet/day and can be taken with or without food vs Mavyret. Mangia A, et al. Liver Int. doi:10.1111/liv.14537.
Counseling Points for Epclusa Can take with or without food. Abstain from sexual intercourse or use condoms. Avoid antacids 4 hours before and 4 hours after taking Epclusa. Monitor for signs of liver problems such as dark urine, light-colored stools, or yellow in the skin or eyes. Can take H2RAs with Epclusa or 12 hours apart.
Monitoring During and After Treatment Perform HCV quantitative RNA test and hepatic function panel at least 12 weeks after completion of treatment. Most patients without cirrhosis won t require lab monitoring. Monitor for side effects throughout treatment. Patients with cirrhosis should continue ultrasound surveillance for hepatocellular carcinoma and upper endoscopic surveillance for varices regularly. Recommend abstaining from alcohol and testing annually for HCV RNA. The American Association for the Study of Liver Diseases and the Infectious Diseases Society of America
What is Cirrhosis? Cirrhosis describes the late stage of progressive liver degeneration, where healthy areas of the liver are slowly replaced by scar tissue (fibrosis). This damage and loss of liver tissue leads to a steady decline in liver function and eventual liver failure. Cirrhosis is the end-stage of this process.
Cirrhosis Diagnosis Several noninvasive techniques such as liver ultrasound (Fibroscan), blood tests (Fibrosure), CT, and MRI. A diagnosis should be made by looking at a patient holistically, considering clinical signs/symptoms, laboratory results, and radiological findings. Gold standard of diagnosis is liver biopsy, although it is not necessary if there is overwhelming evidence of cirrhosis. Uptodate: Cirrhosis in adults: Etiologies, clinical manifestations, and diagnosis
Hep A/B/C viruses Alcohol associated liver disease Common causes of Cirrhosis Non-alcohol related fatty liver disease Hemochromatosis Autoimmune hepatitis Uptodate: Cirrhosis in adults: Etiologies, clinical manifestations, and diagnosis
Complications of Cirrhosis Spontaneous Bacterial Peritonitis (SBP) Varices/Variceal Hemorrhage Ascites Coagulopathies Hepatic Encephalopathy Hepatorenal syndrome Hepatocellular Carcinoma Hepatopulmonary hypertension
Ascites Most common complication of cirrhosis. Defined as a build up of fluid in the peritoneum. Portal hypertension backflow of vasodilatory substances causing splanchnic vasodilation activation of RAAS system excessive sodium and water retention fluid leaks across over-permeable membrane. Patient will present with distended abdomen and uncomfortable bloating. Uptodate: Ascites in adults with cirrhosis: Initial therapy
Ascites Treatment/Prevention Treatment: In severe cases: paracentesis + albumin if more than 20 ml/kg removed Treat the underlying cause: alcohol abstinence or hepatitis virus treatment Sodium restriction of less than 2000 mg/day: fluid follows sodium Spironolactone and Furosemide in the golden ratio of 100:40 mg to offset fluid overload Uptodate: Ascites in adults with cirrhosis: Initial therapy
Esophageal Varices Varices are small, fragile veins that form to bypass the extremely high blood pressure found in the portal artery leading to the liver in people with cirrhosis. These newly formed fragile veins can swell when patients become fluid overloaded due to decompensation. When these blood vessels burst, leads to a life- threatening event called variceal hemorrhage: 15-20% mortality. Most common reason for initial cirrhosis presentation: hematemesis and melena are much more concerning for patients than a distended abdomen. Pubmed: Esophageal Varices
Varices Treatment/Prevention Treatment: Hemodynamic resuscitation and treatment of coagulopathies. 50 mcg bolus of octreotide, followed by 50 mcg/hr continuous infusion for 2-5 days. Prevention Beta blockers such as propranolol decrease portal hypertension and subsequent variceal formation (though NSBBs may be more effective). Variceal banding: a rubber band is surgically inserted around varices which cause them to collapse. Pubmed: Esophageal Varices
Drugs to Avoid in Cirrhosis ACE/ARBS: Particularly avoid in decompensated cirrhosis, ACE/ARBS will lead to an exacerbation in nephrotic hypoperfusion. In these patients, arterial pressure is directly related to survival. Propranolol: Particularly avoid in decompensated cirrhosis: Although useful in preventing varices formation, propranolol specifically has been linked to increased mortality due to reduced blood pressure and contributing to diuretic-resistant ascites. NSAIDs: Inhibition of prostaglandin leads to renal vasoconstriction, exacerbating renal hypoperfusion and can precipitate acute renal failure. Also increases risk of stomach bleeds in these patients with increased risk of coagulopathies. Acetaminophen: Cirrhotic livers have a decreased ability to detoxify NAPQI, limit dose to 2g/day. Uptodate: Ascites in adults with cirrhosis: Initial therapy
Monitoring in Cirrhosis Patients with cirrhosis are screened every 1-3 years for varices, every year if they are decompensated. Patients are screened via ultrasound every 6 months for hepatocellular carcinoma. Regular monitoring of mental status, may order an ammonia level if sudden acute neurological decompensation- could be sign of hepatic encephalopathy. Bi-annual monitoring of kidney function. Pubmed: Surveillance and Diagnosis of Hepatocellular Carcinoma
After confirming a diagnosis of HCV and completing genotyping
Referred pt to hepatologist to determine continued need for furosemide, spironolactone, and propranolol. On 6/13, patient was started on Epclusa 400/100 mg 1 tablet once daily for 12 weeks. MM was counseled on potential side effects such as fatigue, pain management with up to 2 g/day of acetaminophen, and necessary labs. Scheduled to complete 12-week course around 9/5/23, did not discuss during DM follow up on 9/6/23. Will plan to repeat hepatic function test and HCV quantitative RNA test in another 12 weeks.
Diabetes Management 6/13: Switched from glyburide- metformin Synjardy (empagliflozin-metformin) 5-1000 mg BID. 7/5: Increased Synjardy dose to 12.5-1000 mg BID, Started Tradjenta 5 mg. 8/5: Patient self-discontinued Tradjenta due to blood in urine? Patient did not complete Synjardy dose increase (lost in translation). Continued Synjardy 5-1000 mg BID and started Levemir 10 units QHS.
DM: Most recent visit on 9/6 Pt noted concern with increasing Synjardy due to several episodes of increased irritation with urination. Patient noted this only happened a few times and would like to continue for another month at the 5-1000 mg strength. Increased Levemir to 12 units nightly. Currently patient s FBG run around 120-160 and 2-hour PPBG run around 125-190. Still above goal but making great progress!
Current Medication List Medication Strength Dose and Frequency 1 tab BID Indication Synjardy (Empagliflozin and metformin) Levemir Gabapentin Spironolactone Furosemide Propranolol Simvastatin 5-1000 mg Diabetes - 400 mg 100 mg 20 mg 20 mg 20 mg 12 units QHS 1 capsule QHS 1 tablet QD 1 tablet QD 1 tablet BID 1 tablet QHS Diabetes Neuropathy Cirrhosis Cirrhosis Cirrhosis Dyslipidemia
Cirrhosis Medications Medication Strength Dose and Frequency Indication Synjardy (Empagliflozin and metformin) 5-1000 mg 1 tab BID Diabetes Levemir - 12 units QHS Diabetes Gabapentin 400 mg 1 capsule QHS Neuropathy Spironolactone 100 mg 1 tablet QD Cirrhosis Ascites Furosemide 20 mg 1 tablet QD Cirrhosis Propranolol 20 mg 1 tablet BID Cirrhosis Varices Simvastatin 20 mg 1 tablet QHS Dyslipidemia
Future Plans 1 2 3 4 Optimize dose of SGLT- 2 and basal insulin; consider GLP-1 in future. Follow up labs with PCP in November including A1c. Optimize statin, patient is indicated for a high intensity statin based off her age, diabetes, and ASCVD risk enhancers. Follow up with Hep C and LFTs, and work with hepatologist to ensure optimized cirrhosis medications.
Thank you! Questions?
References 1. Fitzmorris P, Singal AK. Surveillance and diagnosis of hepatocellular carcinoma. Gastroenterology & hepatology. January 2015. Accessed September 21, 2023. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4836577/#:~:text=3- ,The%20American%20Association%20for%20the%20Study%20of%20Liver%20Diseases%20(AASLD,(USG)%20every%206%20months. 2. What is viral hepatitis? Centers for Disease Control and Prevention. March 9, 2023. Accessed September 17, 2023. https://www.cdc.gov/hepatitis/abc/index.htm. 3. Hepatitis C virus (HCV) fibrosure . Lapcorp.com. Accessed September 18, 2023. https://www.labcorp.com/tests/related- documents/L9465. 4. M; MM. Esophageal varices. National Center for Biotechnology Information. Accessed September 21, 2023. https://pubmed.ncbi.nlm.nih.gov/28846255/. 5. Ascites in adults with cirrhosis: Initial therapy. UpToDate. Accessed September 21, 2023. https://www.uptodate.com/contents/ascites-in-adults-with-cirrhosis-initial-therapy. 6. Cirrhosis in adults: Etiologies, clinical manifestations, and diagnosis. UpToDate. Accessed September 21, 2023. https://www.uptodate.com/contents/cirrhosis-in-adults-etiologies-clinical-manifestations-and-diagnosis. 7. Sciences G. Epclusa (Gilead Sciences, Inc.): FDA Package Insert, page 2. MedLibrary.org. May 2, 2022. Accessed September 17, 2023. https://medlibrary.org/lib/rx/meds/epclusa/page/2/. 8. Inc. A. Mavyret (AbbVie Inc.): FDA package insert. MedLibrary.org. June 10, 2021. Accessed September 17, 2023. https://medlibrary.org/lib/rx/meds/mavyret/. 9. Hepatitis C - faqs, statistics, resources, find treatment, & more. Centers for Disease Control and Prevention. April 11, 2023. Accessed September 17, 2023. https://www.cdc.gov/hepatitis/hcv/index.htm. 10. What is hepatitis C - FAQ. Centers for Disease Control and Prevention. July 28, 2020. Accessed September 17, 2023. https://www.cdc.gov/hepatitis/hcv/cfaq.htm#E1. 11. Mangia A, Milligan S, Khalili M, et al. Global real world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts. Liver International. 2020;40(8):1841-1852. doi:10.1111/liv.14537