Heterogeneous Catalytic Reaction Steps and LHHW Model Overview
Explore the steps involved in a heterogeneous catalytic reaction and learn about the Langmuir-Hinshelwood-Hougen-Watson (LHHW) model, a common method for deriving rate expressions. Understand the significance of adsorption/desorption processes and how the rate equation accounts for surface phenomena, aiding in accurate extrapolation beyond measured concentrations.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
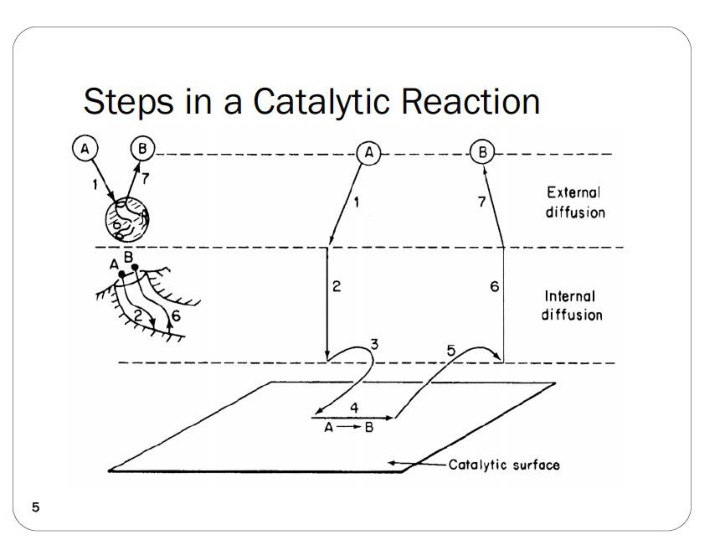
Steps in a Heterogeneous Catalytic Reaction 7. Diffusion of B from external surface to the bulk fluid (external diffusion) 1. Mass transfer of A to surface 2. Diffusion of A from pore mouth to internal catalytic 6. Diffusion of B from pellet interior to pore mouth 3. Adsorption of A onto catalytic surface surface 5. Desorption of product B from surface 4. Reaction on surface Ch 10 assumes steps 1,2,6 & 7 are fast, so only steps 3, 4, and 5 need to be considered
Langmuir-Hinshelwood Hougen- Watson(LHHW) model The approach is one of the most commonly used way of deriving rate expressions for fluid solid catalytic reactions. The advantages of this method are that: (1) Rate derived by this method takes into account the adsorption/desorption process occurring over the surface along with the surface reaction. (2) Rate equation derived can be extrapolated more accurately to concentrations lying beyond the experimentally measured values. Langmuir-Hinshelwood Hougen-Watson(LHHW)
The chemical rate depends on: (1) chemisorption steps (2) surface reaction steps (3) desorption steps In LHHW model development, the rate equation is first derived in terms of surface concentration of adsorbed species and vacant sites. Then, these surface concentrations are related to the fluid or bulk concentration that is directly measurable.
Adsorption Step The adsorption of A (gas phase) on an active site S is represented by: A A I A(g) + S A S -S-S-S- -S-S-S- A S: A bound to a surface site S: open (vacant) surface site Rate of adsorption = rate of attachment rate of detachment r k P C = Molar conc of vacant sites on surface k C Conc of sites occupied by A A S AD A A v A partial pressure of A Rate is proportional to # of collisions with surface, which is a function of PA Rate is proportional to # of vacant (active) sites, Cv, on the surface Active site: site on surface that can form a strong bond with adsorbed species k k A = K In terms of the adsorption equilibrium constant KA where A A k k A = = AD r A A k P C k C AD r k P C C A S A S v A A A v A C A S K = Equation I AD r k P C A A v A
Site Balance Ct: Total number of active sites per unit mass of catalyst divided by Avogadro s # (mol/g cat) Cv: Number of vacant sites per unit mass of catalyst divided by Avogadro s # Active site occupied by A Active site occupied by B Vacant active site A B Surface Cv is not measurable, but the total number of sites Ct can be measured In the absence of catalyst deactivation, assume the total number of active sites remains constant: Site balance: Ct = Cv + CA S + CB S We will use the site balance equation to put Cv in terms of measurable species
Desorption Step Products are desorbed into the gas phase C S C + S = Equation III C C k k P C K D C v = where K D,C r k C D,C I -S-S-S- -S-S-S- CS D D D,C Note that the desorption of C is the reverse of the adsorption of C r = AD,C r D,C Also the desorption equilibrium constant KD,C is the reciprocal of the adsorption equilibrium constant KC 1 = K D,C K C Substituting 1/KC for KD,C in the rate equation for product desorption gives: = D,C r k C C C K P C CS D v
Derive a Rate Law for Catalytic Rxn Postulate catalytic mechanism, and then derive the rate law for that mechanism Assume pseudo-steady state hypothesis (rate of adsorption = rate of surface reaction = rate of desorption) No accumulation of species on the surface or near interface Each species adsorbed on the surface is a reactive intermediate Net rate of formation of species i adsorbed on the surface is 0, ri S=0 One step is usually rate-limiting If the rate-limiting step could be sped up, the entire rxn would be faster Although reactions involve all 7 steps, only adsorption, surface reaction, or desorption will be rate limiting The surface reaction step is rate limiting ~70% of the time! Steps to derive the rate law Select among types of adsorption, surface reaction, and desorption Write rate laws for each individual step, assuming all are reversible Postulate which step is rate limiting Use non-rate-limiting steps to eliminate the surface concentration terms that cannot be measured
Consider A B and assume the following mechanism is correct: C A S K = AD r k P C A(g) + S A S 1. Adsorption: A A v A C C B S K v = S r k C C A S + S S+ B S 2. Surface reaction: A S S v S P C B K v = D r k C B S B + S B S 3. Desorption: D D We need to select one of these 3 reactions as the rate limiting step, then derive the corresponding rate equation, and see if this rate eq matches experimental data. Which step is the most logical to start with? a) Adsorption b) Surface reaction c) Desorption d) None of the above e) Any of these would be logical - they all have equal probability of being the rate limiting step The surface reaction step as is rate limiting ~70% of the time