Honors Environmental Science - Scientific Notation Review & Practice
This resource covers scientific notation concepts, examples, calculations, and answers. It also delves into the International System of Units (SI) for measurements like mass, volume, length, time, and temperature.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Basic Math Pre-assessment Review & Practice Problems Honors Environmental Science Ms. Sipe
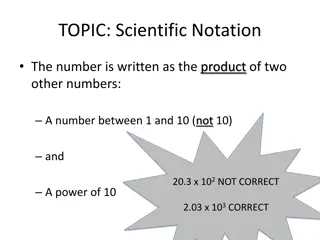
Scientific Notation Scientific notation expresses very large or very small numbers as a multiple of 2 numbers; Based on powers of 10 Ex. 6 x 1023 The 1stnumber The 2ndnumber 1 X < 9 is 10 raised to a power The exponent is the # of places the decimal point must be shifted to give the regular or standard notation. + rt and - lf
Scientific Notation Examples Express the following in scientific notation 0.2713 = 314159 = 6022 = 0.12011 = Express the following in standard or expanded form 4.44 x 10-6= 7.121 x 109= 1.2 x 10-1= 1.8 x 102=
Scientific Notation Answers Express the following in scientific notation 0.2713 = 2.713 X 10-1 314159 = 3.14159 X 105 6022 = 6.022 X 103 0.12011 = 1.2011 X 10-1 Express the following in standard or expanded form 4.44 x 10-6= 0.00000444 7.121 x 109= 7,121,000,000 1.2 x 10-1= 0.12 1.8 x 102= 180
Scientific Notation Calculations To X exponential terms add the powers; to / exponential terms subtract. To + or exponential terms, the exponents must be the same. Solve the following: a. (3.0 x 10-8)(6.0 x 102) = b. (2.0 x 104) (1.0 x 1013)= c. (2.0 x 102) + (3.0 x 103) = d. (2.5 x 103)(2.0 x 10-7) = e. (7.0 x 101) = (3.5 x 10-3) f. (5.5 x 103) - (5.0 x 102) =
Scientific Notation Calculations Answers To x exponential terms add the powers; to / exponential terms subtract. To + or exponential terms, the exponents must be the same. Then, put the answer in proper scientific notation. Solve the following: a. (3.0 x 10-8)(6.0 x 102) = 1.8 X 10-5 b. (2.0 x 104) (1.0 x 1013)= 2.0 X 10-9 c. (2.0 x 102) + (3.0 x 103) = 3.2 X 103 d. (2.5 x 103)(2.0 x 10-7) = 5.0 X 10-4 e. (7.0 x 101) = 2.0 X 104 (3.5 x 10-3) f. (5.5 x 103) - (5.0 x 102) = 5.0 X 103
SI -The international system of units Base Unit need only one measurement Derived Unit a combination of 2 or more base units Quantity Typical Unit SI Unit Base unit (B) or Derived unit (D) Equipment Definitions Mass Measure of the amount of matter g g B Triple beam balance Volume Measure of the Space that matter takes up mL D Graduated cylinder g/cm3or g/ml Density ratio of the mass of an object to its volume. D Length distance between two points m m B Ruler Time Duration of an event s s B Stop watch, clock, timer Temperature Measure of KE (vibration of molecules) C K B thermometer
The Metric System and Prefixes / 103 102 101 100 10-1 10-2 10-3 X
The Metric System and Prefixes Also should also know: Tera (T) 1012 Giga (G) 109 Mega (M) 106 Micro (u) 10-6 Nano (n) 10-9 Pico (p) 10-12 METRIC CONVERSIONS USING DIMENSIONAL ANALYSIS: 1,000,000 g = 1g 1,000mg = 1 g 100cg = 1 g 10dg = 1 g 1 kg = 1,000g 1,000,000 L = 1 L 1,000mL = 1 L 100cL = 1 L 10dL = 1 L 1 kL = 1,000 L
Metric Conversions 1. 876 milliliters to liters 5) 11.7 grams to kilograms 2. 78,999 milligrams to grams 6) 0.0009 kiloliters to liters 3. 0.9 centigrams to grams 7) 44 centimeters to meters 4. 112 meters to millimeters 8) 277 kilograms to grams
Metric Conversion Answers 1. 876ml(1L/1000mL) = 0.876 L 876 milliliters to liters 5) 11.7 grams to kilograms 11.7g (1 kg/1000g) = 0.011700 2. 78,999 milligrams to grams 78,999mg(1g/1000mg) = 78.999 g 6) 0.0009 kiloliters to liters 0.0009kL(1000L/1kL) =0.9 L 3. 0.9 centigrams to grams 0.9cg(1g/100cg)0.009 g 7) 44 centimeters to meters 44cm(1m/100cm) = 0.44 m 4. 112 meters to millimeters 112m(1000mm/1m) = 112,000 mL 8) 277 kilograms to grams 277kg(1000g/1kg)= 277,000 g
Dimensional Analysis What s given? What do you want? Look for an equivalence statement to put into ratio form Show all work Set-up (bridge) with all units May have one or multiple steps Did you answer the question? Is the answer reasonable?
Dimensional Analysis Practice Given: 2.54 cm = 1 inch 3.79 L = 1 gallon 16 ounces = 1 pound 1 L = 1000 mL Solve: 5.7 inches = _____________ cm 125 grams = ______________ lbs 2.5 miles = ______________ feet 1 mile = 5280 feet 454 g = 1 pound 1 calorie = 4.18 Joules 1 mole = 6.02 X 1023 atoms 1 gallon = 4 quarts 1 ft = 12 inches 1 mL = 1 cm3 85 ounces = ________________ lbs 72 cm3 = ______________ mL 4 moles = ________________ atoms
Dimensional Analysis Answers Solve: 5.7 inches = _____________ cm 5.7in(2.54cm/1in) = 14.478cm 85 ounces = ________________ lbs 85oz(1lb/160z) = 5.31lbs 72 cm3 = ______________ mL 72 cm3(1mL/1cm3)= 72mL 125 grams = ______________ lbs 125g(1lb/454g) = 0.28lbs 2.5 miles = ______________ feet 2.5mi(5280ft/1mi)= 13200ft 4 moles = ________________ atoms 4mol(6.022X 1023 atoms/1mol)= 2.41 X 1024 atoms
More to Come: Nuclear Chemistry reactions & calculations. Concentration reactions & calculations