How to Write Compound Formulas: Ionic, Covalent, Polyatomic
Learn how to write compound formulas for binary ionic, multivalent, polyatomic, and covalent compounds. Understand the naming conventions for each type of compound and how to balance charges to form the correct formula.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
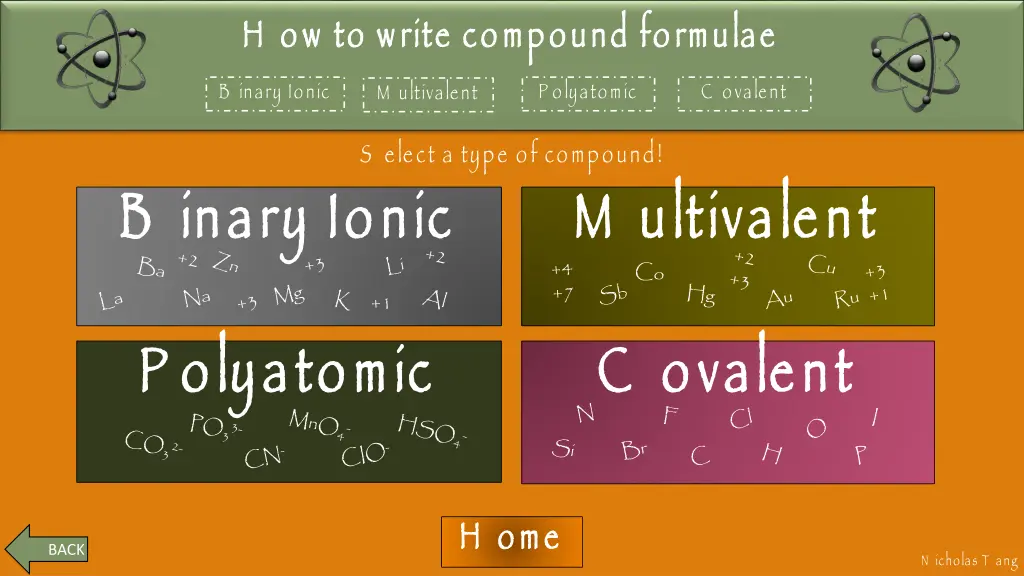
How to write compound formulae H ow to write compound formulae M ultivalent H ow to write compound formulae Binary Ionic Polyatomic Covalent Multivalent B inary Ionic P olyatomic C ovalent S elect a type of compound! B inary Ionic B inary Ionic M ultivalent M ultivalent P olyatomic P olyatomic C ovalent C ovalent H ome H ome BACK BACK BACK N icholas T ang
How to write compound formulae H ow to write compound formulae M ultivalent H ow to write compound formulae Binary Ionic Polyatomic Covalent Multivalent B inary Ionic P olyatomic C ovalent A binary ionic compound is made of 2 elements, a metal and a non-metal, where both elements have only one ion charge (+ 1 , + 2 , + 3 ) S ome examples are: S odium (N a), P otassium (K ) and A luminium (A l). In all compounds, the positive ion (usually a metal) is named first and the negative ion (non-metal) is named second. E xample: M agnesium N itride M g2 + N3 - M g2 + N3 - M g2 + Y ou need 3 M agnesium and 2 N itrogen to balance even out M g3N2 M g3N2 B inary Ionic C ompounds B inary Ionic C ompounds BACK BACK BACK N icholas T ang
How to write compound formulae H ow to write compound formulae M ultivalent H ow to write compound formulae Binary Ionic Polyatomic Covalent Multivalent B inary Ionic P olyatomic C ovalent M ultivalent compounds are made of a metal and a non-metal. It is called multivalent because it the metal has more than 1 electrical charge (+ 1 , + 2 ). In order to identify the ion charge, there will be a roman numeral next to the name: gold (III) oxide, copper (I) fluoride E xample: platinum (II) nitride P t2 + N3 - P t2 + N3 - P t2 + N3 - x 2 = 6 It evens out on both sides 1 I 2 II 3 III 4 IV 5 V 6 V I 7 V II 8 V III 9 IX 1 0 - X It could also be P t3N4 if the name was platinum (IV ) nitride P t3N2 P t3N2 P t2 + x 3 = 6 M ultivalent C ompounds M ultivalent C ompounds BACK BACK BACK N icholas T ang
How to write compound formulae H ow to write compound formulae M ultivalent H ow to write compound formulae Binary Ionic Polyatomic Covalent Multivalent B inary Ionic P olyatomic C ovalent P olyatomic compounds are compounds that contain a polyatomic ion. P olyatomic ions are created when a number of atoms come together to form a group, and then that group can have either a positive or a negative charge. S ome examples are: N itrate (N O3-), A mmonium (N H4+) and H ydroxide (O H-). E xample: C alcium N itrate C a2 + N O3- N O3- C a(N O3)2 C a(N O3)2 P olyatomic C ompounds P olyatomic C ompounds BACK BACK BACK N icholas T ang
How to write compound formulae H ow to write compound formulae M ultivalent H ow to write compound formulae Binary Ionic Polyatomic Covalent Multivalent B inary Ionic P olyatomic C ovalent C ovalent compounds are compounds made of two or more non-metals. T hey are made when two or more non-metals share electrons and the bond that is created by sharing electrons is called a covalent bond. S ome examples are: O xygen (O ), F luorine (F ) and N itrogen (N ). E xample: C arbon D ioxide C+ 1 O2 - O2 - C ovalent C ompounds 1 mono 2 - di 3 tri 4 tetra 5 hexa 7 hepta 8 octa 9 nono 1 0 - deca C arbon D ioxide C O2 C O2 If you have ao or oo, turn it into o: heptoxide C ovalent C ompounds BACK BACK BACK N icholas T ang