Hybridisation in Chemistry
Explore the concept of hybridisation in chemistry, including its rules, types like sp and sp2, characteristics, and examples such as acetylene and ethylene. Discover how hybrid orbitals form sigma bonds and contribute to molecular structure.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
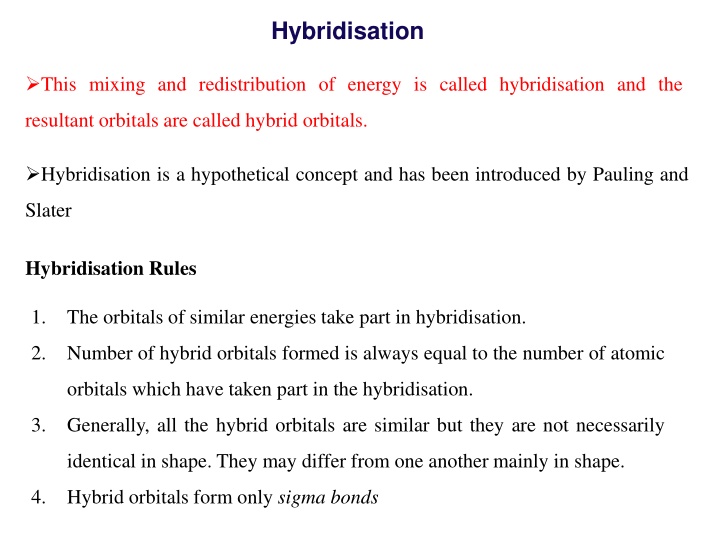
Hybridisation This mixing and redistribution of energy is called hybridisation and the resultant orbitals are called hybrid orbitals. Hybridisation is a hypothetical concept and has been introduced by Pauling and Slater Hybridisation Rules 1. The orbitals of similar energies take part in hybridisation. 2. Number of hybrid orbitals formed is always equal to the number of atomic orbitals which have taken part in the hybridisation. 3. Generally, all the hybrid orbitals are similar but they are not necessarily identical in shape. They may differ from one another mainly in shape. 4. Hybrid orbitals form only sigma bonds
sp or Digonal Hybridisation : In this type of hybridisation one s and one p-orbital of the valence shell of central atom of the given molecule combine to form two sp hybrid orbitals as follows:
Characteristic of sp-Hybrid Orbitals 1. Both sp-hybrid orbitals are completely equivalent and symmetrical. 2. Energy of sp-hybrid orbital is more than s-orbital but less than the p- orbital. 3. These two sp-hybrid orbitals are collinear, i.e., angle between the hybrid orbitals is 180 . 4. Shape of sp-hybrid orbital is oval. 5. Its relative power of overlapping is 1.93 with respect to s orbital. 6. In sp-hybrid orbital one lobe is bigger while other lobe is small. The bigger lobe is very large with respect to p-orbital, hence it has higher degree of overlapping. Thus it forms stronger bond.
sp2 or Trigonal Hybridisation : In this type of hybridisation one s and 2p orbitals of the valence shell of central atom of the given molecule combine to form three sp2-hybrid orbitals as shown below:
Characteristics of sp2 Hybridisation : (1) These sp2-hybrid orbitals are completely equivalent and symmetrical. (2) These hybrid orbitals are planar with bond angle 120 . (3) Since in this hybridisation contribution of p-orbitals is more hence it is less oval than sp-hybrid orbitals. In this case one lobe is bigger and one lobe is smaller and it forms stronger bond. (4) These are stronger than s and p orbitals. Its relative power of overlapping is 1.99 with respect to s-orbital
sp3 or Tetrahedral Hybridisation: In this hybridisation one s and three p-orbitals of the valence shell of central atom of the given molecule combine to form four sp3-hybrid orbitals.
Characteristics of sp3 Hybridisation : (1) All the four sp3 hybrid orbitals are completely equivalent and symmetrical. (2) These orbitals are directed towards the four comers of a regular tetrahedron and the angle between each pair of them is 109 28' or 109.5 . (3) Their relative power of overlapping is 2.00 with respect to s-orbital. This shows that sp3 -orbitals are stronger than sp which is stronger than sp- orbitals. (4) Since in sp3-hybridisation the contribution of p-orbitals is 75%, its shape is almost same as that of the parent p-orbitals except that the bigger lobe in sp3 -orbital is somewhat more spread and shorter in length than the pure p- orbitals
Hybridisation in Organic Species Hybridisation in organic species can be known by two methods: First Method: In this method hybridisation can be known by the number of pi bonds present on that particular atom. Example This method cannot be used for those atoms of the molecule which have positive charge, negative charge or odd electron
Second method: Electron pair method ep = bp + lp where ep = electrons pair present in hybrid orbitals bp = bond pair present in hybrid orbitals lp = loan pair present in hybrid molecule Determination of bond pairs : Number of bp = Number of atoms present on central atom of the species
Determination of lone pair of electrons: Number of Ip's can be determined as follows: (i) If carbon has pi bond/(s) or positive charge or odd electron, then lp on carbon will be zero. (ii) If carbon has negative charge, then Ip will be equal to one. Number of electron pairs tells us the type of hybridisation as follows:
