Hydrates in Chemistry
Learn about hydrates in chemistry, including their structure, removal of water molecules, naming conventions, and examples. Discover how to determine water content in hydrates through experiments and calculations.
Uploaded on | 1 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
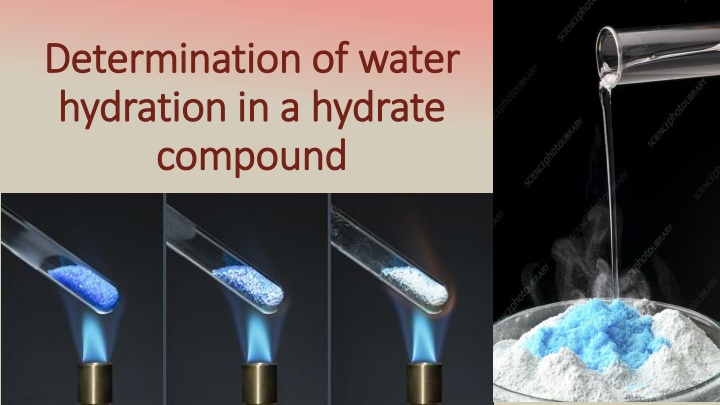
Determination of water Determination of water hydration hydration in a hydrate in a hydrate compound compound
WHAT IS HYDRAT WHAT IS HYDRAT A Hydrate is an ionic compound that contain water molecules in its fundamental solid structure called waters of hydration. Waters of hydration can be removed by heating.
Anhydrous compounds are chemical compounds that have remove water molecules in the chemical structure.
Naming hydrates Naming hydrates Some molecule attaches with water molecules ,this is done set numbers depended of molecules. while named by (Write compound and hydrate connected by the dot). Example /Gypsum is a hydrate in which two water molecules are present for every formula unit of CaSO4 in the solid. The chemical formula for gypsum is CaSO4 2 H2O and the chemical name is calcium sulfate dehydrate. .
copper (II) sulphate pentahydrate, magnesiumsulphate heptahydrate, cobalt (II) chloride hexahydrate tin (II) chloride dihydrate
Percent error = ( theoretical - experimental / theoretical value) x 100
Example : When a 1.000 g sample of CuSO4 5 H2O(s) was heated so that the waters of hydration were driven off, the mass of the anhydrous salt remaining was found to be 0.6390 g. A-What is the experimental value of the percent water of hydration? B- Calculate the percent error. ( Cu = 63.55 , S = 32 , O =16 , H =1 g / mol)
Procedure: Procedure: Weight a pure, dry and empty crucible. put 1g of copper(II) sulphate pentahydrate (hydrate compound)in the crucible. heat the crucible and its content for half hour(the color change from blue to white and finally to grey) . Cool in desiccators. Weight the crucible with anhydrous compound. Calculate the percentage of water in that sample. Write all Chemical Compounds and all Apparatus used in this experiment.






















