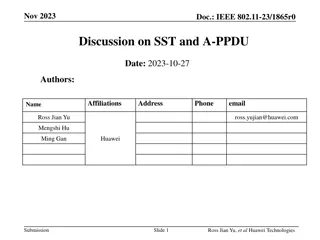
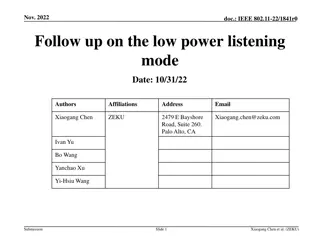
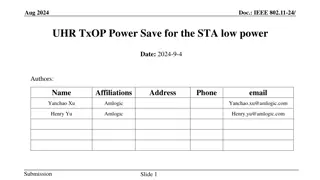
IEEE 802.11-23/1942r0 Inter-PPDU Low Power Listening Scheme
Reducing power consumption in WLAN devices is vital for IEEE 802.11bn networks. This document discusses an Inter-PPDU Low Power Listening (LPL) scheme aimed at efficient power management by minimizing signaling overhead and latency related to changing PHY parameters. The scheme introduces a method for transitioning from low power (LP) mode to high power (HP) mode without extensive padding, ensuring minimal disruption to other stations. Details include the use of Notification PPDU for wake-up indications and delivering data to target STAs.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
OVERVIEW OF HYDRATE CALCULATIONS CALCULATION OF THE WATER OF HYDRATION COEFFICIENT 1. FROM YOUR DATA, FIND MASS OF ANHYDROUS SALT ( SUBTRACT EMPTY BEAKER FROM BEAKER CONTAINNING THE ANHYDRATE AT END OF LAB). 1. CALCULATE THE MOLAR MASS (GFM) OF YOUR ANHYDROUS SALT AND CONVERT YOUR MASS TO MOLES. 2. FROM YOUR DATA, FIND MASS OF WATER LOST DURING HEATING (SUBTRACT THE MASS OF THE BEAKER CONTAINNING HYDRATE BEFORE HEATING FORM BEAKER AFTER HEATING). 3. CALCULATE THE MOLAR MASS OF WATER AND CONVERT THE WATER TO MOLES. 4. COMPARE THE MOLES OF ANHYDROUS SALT TO MOLES OF WATER BY DIVIDING BY THE SMALLEST, THIS SHOULD GIVE THE WATER OF HYDRATION COEFFICIENT.
SAMPLE DATA for dehydration of CuSO4. X H2O EMPTY BEAKER = 70.0 g 0.70 GRAMS WATER WAS LOST DURING HEATING. BEAKER + HYDRATE = 72.0 g To calculate the water mass, subtract the beaker before heating (contains hydrated salt, from the beaker after heating (contains the anhydrous salt). The mass lost is water gas that escaped your beaker BEAKER AFTER FINAL = 71.3 g HEATING (CONTAINS ANHYDROUS SALT) 71.3g - 70.0 g = 1.3 g OF ANHYDROUS SALT (CuSO4 ) To calculate the mass of the anhydrous ( without water ) salt simply subtract the empty beaker from the beaker after heating. After the heating cycle the hydrate has been dehydrated and is Termed the anhydrous salt.
CALACULATE THE MOLAR MASS OF THE ANHYDROUS SALT CuSO4 Subtotal for the element element Atomic mass X Cu 1 63.546 = 63.546 X S 1 32.06 = 32.06 X O 4 15.99 = 63.96 + 159.566g/mol Formula subscripts
CALACULATE THE MOLAR MASS OF THE H2O Formula subscripts Subtotal for the element 2.01588 element Atomic mass X H 2 1.00794 = X O 1 15.99 = 15.99 + 18.00588 g/mol
CALACULATE THE MOLES OF THE H2O element Subtotal for the element Atomic mass X 2.01588 H 2 1.00794 = X O 1 15.99 = + 15.99 18.00588 g/mol MOLES = MASS/GFM MOLES (H2O) = 0.70g/18.00588 g/mol MOLES (H2O) = 0.0388761 mol H2O in hydrate
CALACULATE THE MOLES THE ANHYDROUS SALT CuSO4 Subtotal for the element element Atomic mass X 63.546 Cu 1 63.546 = X S 1 32.06 = 32.06 X O 4 15.99 = 63.96 + 159.566g/mol MOLES = MASS/GFM MOLES (CuSO4) = 1.3g/156.566 g/mol MOLES (CuSO4) = 0.0083037 mol CuSO4 in hydrate
MOLES (H2O) = 0.0388761 mol H2O in hydrate MOLES (CuSO4) = 0.0083037 mol CuSO4 in hydrate Now that we have the moles of the water and anhydrous salt, we divide by the smallest number of moles to get in integer ratio. 0.0388761 mol H2O = 4.68 which rounds to 5 0.0083037 mol CuSO4 Therefore the mole ratio of water to anhydrous salt is 5:1, X, the water of hydration is 5.