Immunochromatography and Its Components
Immunochromatography is a technique combining chromatography and immunochemical reactions to detect target analytes. The process involves a test strip with components like a sample application pad, conjugate pad, nitrocellulose membrane substrate, and adsorbent pad. Learn more about the development and working principle of Immunochromatography assays.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
chromatography generally refers to procedures adapted to separate and characterise substances on the basis of their size, ionic charge or stability in particular solvents. Immunochromatography assay (ICA), namely lateral flow test, is a simple device intended to detect the presence or absence of the target analyte. The concept of immune-chromatography is a combination of chromatography (separation of components of a sample based on differences in their movement through a sorbent) and immunochemical reactions. The most widespread immunochromatographic system is the test strip
A one-step qualitative immunochromatographic procedure has been introduced in serodiagnosis.because of simplicity,economy and reliability of the test, it has wide acceptance. the principle of the test the procedure for detection of HBsAg. the test system consists of a small casette containing a membrane impragnated with anti-HBsAg antibody-colloidal gold dye conjugate. the memrane is exposed at three window cassette. serum to be tested is dropped into the first window of the cassatte. the second window contains the anti-HBsAg antibody colloidal gold dye conjugate. As the test serum moves upstream by capillari action, a coloured band developes at the second window(test site)if the serum contain HBsAg- as a result formation of HBsAg antibody conjugate complex. A negative reaction does not produce a coloured band at the test site. simultaneously,a coloured band should develop in every case at third window,which form an inbuilt conrol. The test is sensitive and specific as immunoassays.
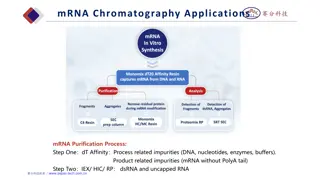
Developing an ICA Strips used for ICA contain four main components: 1. Sample Application Pad It is made of cellulose and/or glass fiber and sample is applied on this pad to start the assay. Its function is to transport the sample to other components. Sample pad should be capable of transportation of the sample in a smooth, continuous and homogenous manner. This pretreatment may include separation of sample components, removal of interferences, adjustment of the pH, etc. analyte sample should be added to the sample application pad to start the test. 2. Conjugate Pad It is the place where labeled biorecognition molecules (labeled antibodies, usually nano colloid gold particle) are dispensed. Material of conjugate pad should immediately release labeled conjugate upon contact with moving liquid sample. Labeled conjugate should stay stable over entire life span of the lateral flow strip. Any variations in dispensing, drying or release of conjugate can change the results of assay significantly. Poor preparation of labeled conjugate can adversely affect sensitivity of the assay. Glass fiber, cellulose, polyesters and some other materials are used to make
3. Substrate(Nitrocellulose) Membrane It is highly critical in determining sensitivity of ICA. Test and control lines are drawn over this piece of membrane. So an ideal membrane should provide support and good binding to capture probes (antibodies, etc.). Nonspecific adsorption over test and control lines may affect results of assay significantly, thus a good membrane will be characterized by lesser non-specific adsorption in the regions of test and control lines. Proper dispensing of bioreagents, drying and blocking play a role in improving sensitivity of the assay. 4. Adsorbent Pad It works as sink at the end of the strip. It also helps in maintaining flow rate of the liquid over the membrane and stops back flow of the sample. Adsorbent capacity to hold liquid can play an important role in results of assay. All these components are fixed or mounted over a backing card. Materials for backing card are highly flexible because they have nothing to do with ICA except providing a platform for proper assembling of all the components. Thus, backing card serves as a support and it makes easy to handle the strip.
ICA Format Lateral flow assay basically combines a number of variants such as formats, biorecognition molecules, labels, detection systems and applications. There are three types of ICAs based on detection format, which are: 1. Sandwich Assay In this assay format, label (Enzymes or nanoparticles or fluorescence dyes) coated antibody is immobilized at conjugate pad. This is a temporary adsorption which can be flushed away by flow of any buffer solution. A capture antibody against target analyte is immobilized over test line. A secondary antibody against labeled antibody is immobilized at control zone (Figure 2).
2. Competitive Assay Competitive format has two layouts. In the first layout, solution containing target analyte is applied onto the sample application pad and prefixed labeled antibody gets hydrated and starts flowing with moving liquid. Test line contains pre-immobilized antigen (same analyte to be detected) which binds specifically to label conjugate. Control line contains pre-immobilized secondary antibody which has the ability to bind with labeled antibody. When liquid sample reaches at the test line, pre-immobilized antigen will bind to the labeled conjugate in case target analyte in sample solution is absent or present in such a low quantity that some sites of labeled antibody conjugate were vacant. Antigen in the sample solution and the one which is immobilized at test line of strip compete to bind with labeled conjugate (Figure 3.). 3. In another layout, labeled analyte conjugate is dispensed at conjugate pad while a primary antibody to analyte is dispensed at test line. After application of analyte solution, a competition takes place between analyte and labeled analyte to bind with primary antibody at test line. Such format suits best for low molecular weight compounds which cannot bind two antibodies simultaneously. Absence of color at test line is an indication for the presence of analyte while appearance of color both at test and control lines indicates a negative result.
3. Multiplex Detection Assay Multiplex detection format is used for detection of more than one target species and assay is performed over the strip containing test lines equal to number of target species to be analyzed. It is highly desirable to analyze multiple analytes simultaneously under the same set of conditions. Multiplex detection format is very useful in clinical diagnosis
Principle: fluorescence is the property of certain dyes,which absorb light in the ultraviolet region and emit a characteristic wave length of light in the visible region boons and kaplan,who showed that fluorescent labelled protiens and localisation and identification of antigen in tissue in the body. e.g. fluorescine isothiocynate exhibit blue green rhodamine orange red fluorescent Procedure: immunofluorescent tests- reffered to as fluorescent antibody tests(FAT)- are of two types 1. Direct immunofluorescence 2. Indirect immunofiorescence
IF type Direct Indirect Combined Schematic diagram Rapid staining Available for antibodies from the same host Secondary signaling amplification A single secondary antibody can detect multiple primary antibodies from the same host Secondary amplification for weak signaling Available for antibodies from the same host Advantages Less sensitive because of lack of secondary signaling amplification Time-consuming Require antibodies from different hosts Possibility of antibody cross-reactivity Multiple-step staining Disadvantages
1. Direct immunofluorescence this technique also known as Direct Fluorescence Antibody(DFA)test, is the bsimplest form of immunofluorescence. Blood film or smear is fixed in 95%ethanol for 5 minutes and dried. Then fluorescein-tagged(isothiocyanate)antibodies are brought into contact with antigen fixed on the slide allowing them to react by incubation at 37 for half an hour. The exccess off antibody is washed off and the preparation The exccess off antibody is washed off and the preparation is eaxamined under the ultraviolet light microscope is eaxamined under the ultraviolet light microscope e.g. of DFA method is testing of urogenital specimen for e.g. of DFA method is testing of urogenital specimen for chlamydia in nongonococcal urethritis. chlamydia in nongonococcal urethritis. the specimen is placed in the well of a fluorescent the specimen is placed in the well of a fluorescent antibody slide and reacted with a fluorescein antibody slide and reacted with a fluorescein- -labelled monoclonal antibody directed against chlamydia. monoclonal antibody directed against chlamydia. After incubation and washing the slide will show After incubation and washing the slide will show characteristic fluorescence characteristic fluorescence- -if positive for chlamydia. if positive for chlamydia. labelled
Uses: this simple technique is commonly employed in the diagnostic laboratory for identification of bacteria, viruses, or other antigen in tissue sections, blood, CSF, urine, feaces and other specimens. Disadvantage: separate fluorescent-tagged antibody has to be prepared against each antigen to be tested.
2 Indirect immunofluorescence(IF) this test is useful in detection of antibodies in fluorescent antibofy(IFA) in serum or other body fluids. this is double layer technique. IFA test may also be applied in antigen detection. Indirect FAT to detect antibody 1. -The unlabelled unknown antibody is applied to the known antigen on slide such as T.paliiidum. -After incubation the slide is washed carefully with buffer to remove unfixed excess serum leaving only antibody globulin which coats of the antigen(e.g treponomas). 2. -The smear is treated with a fluorescein tagged antibody to humman globulin. - the slide is washed to remove excess fluorescein conjugate and examined under UV light. in positive case the Ag-Ab complex will fluoresce
slide preparation of specimen (Ag) is made and unlabelled specific known Ab is added. After incubation the slide is washed with buffer leaving only Ab that has combined with the Ag on the slide. A fluorescent labelled anti-species globulin (AHG, anti-human globulin produced in goat or rabbit)is added and allowed to combine with the Ab.Excess AHG is washed off from the slide and examined under UV light. In positive case the Ag-Ab cpmlexes will be seen fluorescing brightly due to the attachedfluorescent labelled antiglobulin. the indirect FAT is often used in preference to direct FAT for demonstration of an antigen in a specimen because it allows the antiserum (a single antihuman globilin fluorescent conjugate)(unlabelled) for use in other serological test.