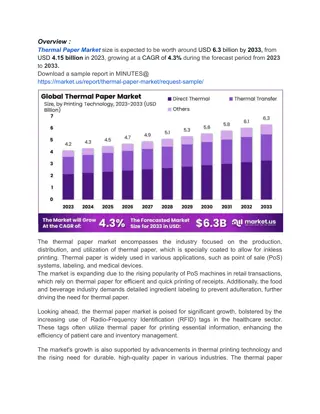
Infrared Thermography and Emissivity in Heat Transfer
Explore the concepts of Infrared Thermography, heat transfer methods, and emissivity in this informative section. Discover how thermal imaging captures radiation, the significance of thermodynamics laws, and the impact of surface temperature variations. Uncover the relationship between energy emission and material states, and grasp the importance of emissivity in radiative energy transfer.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
In this section you will learn What Infrared Thermography is Methods of heat transfer What emissivity is and how it affects thermography
Infrared thermography (IRT), thermal imaging, and thermal video are examples of infrared imaging science. 2
Thermal imaging cameras detect radiation in the infrared range of the electromagnetic spectrum and produce images of that radiation, called thermograms.
Since infrared radiation is emitted by all objects, thermography makes it possible to see one's environment with or without visible illumination. The amount of radiation emitted by an object increases with temperature; therefore, thermography allows one to see variations in surface temperature.
Laws of Thermodynamics Conduction Convection Heat Radiation 5
Laws of Thermodynamics 1st -Energy can be changed from one form to another, but it cannot be created or destroyed. (Conservation) 2nd - in all energy exchanges, if no energy enters or leaves the system, the potential energy of the state will always be less than that of the initial state. (Entropy) 6
Materials exist in one of three states. These are solid, liquid and gas. In order to change the state of a material, energy must be added or removed. 7
Heat is added as change occurs in this direction While in this direction heat is removed 8
Emissivity- is the relative ability of an objects surface to emit energy by radiation. 10
High Emissivity You can trust what you see Emissivity Demonstration Low Emissivity You can t trust what you see 11
In general, the duller and blacker a material is, the closer its emissivity is to 1. The more reflective a material is, the lower its emissivity. Highly polished silverhas an emissivity of about 0.02.
Emissivity depends on factors such astemperature, emissionangle, andwavelength. A typicalphysicalassumption is that a surface's spectral emissivity and absorptivitydo not depend on wavelength, so that the emissivity is a constant. This is known as the "gray body assumption". 13
Reflectivity - is inversely related to emissivity. When added together, the emissivity and reflectivity will equal 1. Gypsum has an emissivity of .85 which means it has a reflectivity of .15. That means that when you point an infrared camera at sheetrock you are reading 85% emitted and 15% reflected energy. 14
Reflection Types Reflectivity Demonstration Specular Diffuse